Abstract
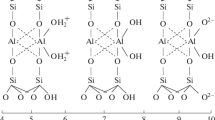
Suspension of a Tunisian purified smectite and American montmorillonite are studied by acid-base potentiometric and mass titrations. These experimental methods are used to determine the point of zero net proton charge (PZNPC). A very good agreement is observed between the two kinds of experiments. The two Namontmorillonites, studied at different ionic strengths, present proton adsorption vs. pH curves with a common crossing point. The PZNPC of the edge sites are 8.02 for Tunisian purified smectite and 8.11 for pure American montmorillonite. By analyzing the proton adsorption or desorption (H+ vs. pH) curves, one may assume the presence of four active sites at the surface. The montmorillonite surface undergoes two successive protonations and two successive deprotonations. Below pH < PZNPC and in acidic range, the cation exchange at layer sites and protonation of edge sites (>A1OH groups) occur simultaneously. For pH > PZNPC and in alkaline pH range, deprotonation of surface hydroxyl groups exposed at the edge sites (>SiOH, and >A1OH at high pH) of montmorillonite platelets causes an overall negative charge.
Similar content being viewed by others
References
Wanner, H., Albinson, Y., Karnland, O., Wicland, E., Wcrsin, P., and Chariot, L., Radiochim. Acta, 1994, vol. 157, p. 66.
Frenkel, M., Clays Clay Miner., 1974, vol. 22, p. 435.
Svcrjcnsky, D., Geochim. Cosmochim. Acta, 2005, vol. 69, p. 225.
James, R.O. and Parks, G.A., Surf. Colloid Sci., 1982, vol. 12, p. 119.
Hayes, K.F., Redden, G., Ela, W., and Leckie, J.O., J. Colloid Interface Sci., 1991, vol. 142, p. 2.
Wicland, E. and Stumrn, W., Geochim. Cosmochim. Acta, 1992, vol. 56, p. 3339.
Sprycha, R. and Colloid, J., Interface Sci., 1989, vol. 127, p. 1.
Du, Q., Sun, Z.X., Forsling, W., and Tang, H.X., J. Colloid Interface Sci., 1997, vol. 187, p. 221.
Tonibacz, E. and Szekers, M., Langmuir, 2001, vol. 17, p. 5.
Sposito, G., The Surface Chemistry of Soils, Oxford: Oxford University Press, 1984.
Avena, M.J., Cabrol, R., and de Pauli, C.P., Clays Clay Miner., 1990, vol. 38, p. 356.
Avena, M.J. and de Pauli, C.P., J. Colloid Interface Sci., 1998, vol. 202, p. 195.
Missana, T. and Adell, A., J. Colloid Interface Sci., 2000, vol. 230, p. 150.
Kraepiet, A.M.L., Keller, K., and Morel, F.M.M., J. Colloid Interface Sci., 1999, vol. 210, p. 43.
Schroth, B.L. and Sposito, G., Clays Clay Miner., 1997, vol. 45, p. 85.
Zhuang, J. and Gui-Rui, Y., Chemosphere, 2002, vol. 49, p. 619.
Itami, K. and Fujitani, H., Colloids Surf. A, 2005, vol. 265, p. 55.
Appel, C., Lena, Q.M., Rhue, R.D., and Kenncllcy, E., Geoderma, 2003, vol. 113, p. 77.
Noh, J.S. and Schwarz, J.A., J. Colloid Interface Sci., 1989, vol. 130, p. 1.
Tsuchida, H., Ooi, S., Nakaishi, K., and Adaclii, Y., Colloids Surf., A, 2005, vol. 265, p. 131.
Blok, L. and de Bruyn, P.L., J. Colloid Interface Sci., 1970, vol. 32, p. 3.
Duc, M., Gaboriaud, F., and Thomas, F., J. Colloid Interface Sci., 2005, vol. 289, p. 148.
Spreacha, R., Jablonski, J., and Matijevik, E., J. Colloid Interface Sci., 1992, vol. 149, p. 561.
Chorover, J., Amustadi, M.K., and Cliadwick, O.A., Geochim. Cosmochim. Acta, 2004, vol. 68, p. 4859.
De Faria, L.A. and Trassati, S., J. Colloid Interface Sci., 1994, vol. 167, p. 352.
Ganor, J., Cama, J., and Metz, V., J. Colloid Interface Sci., 2003, vol. 264, p. 67.
Bleam, W.F., Welhouse, G.J., and Janowiak, M.A., Clays Clay Miner., 1993, vol. 41, p. 305.
Bleam, W.F., Clays Clay Miner., 1990, vol. 38, p. 527.
Madrid, L. and Diaz-Barrientos, E., J. Soil Sci. Soc., 1988, vol. 39, p. 215.
Hendershot, W.H. and Lavkulich, L.M., Soil Sci. Soc. Am. J., 1983, vol. 47, p. 1252.
Helmy, A.K., Ferreiro, E.A., and De Bussctti, S.G., Clays Clay Miner, 1994, vol. 42, p. 444.
Tombacz, E., Abraham, I., Gilde, M., and Szanclo, F., Colloids Surf., 1994, vol. 4, p. 71.
Stadler, M. and Schindler, P.W., Clays Clay Miner., 1993, vol. 41, p. 288.
Sposito, G., Environ. Sci. Technol., 1998, vol. 32, p. 2815.
Bourikas, K., Kordulis, C., and Leycourghiotis, A., Environ. Sci. Technol., 2005, vol. 39, p. 4100.
Huertas, F.J., Chou, L., and Wollast, R., Geochim. Cosmochim. Acta, 1998, vol. 62, p. 417.
Srasra, E., Ariguib, N., Bergaya, F., and van Damme, H., in Communication a la Societe Chimique de Tunisie, Quatriemejournee de Chimie, Hammamet, Nov. 21–23, 1986.
Olphen, V., An Introduction to Clay Colloid Chemistry, New York: Interscience, 1963.
Bergaya, F. and Vayer, M., Appl. Clay Sci., 1997, vol. 12, p. 275.
Boissay, S., Comparison des méthodes de détermination des points de charge nulle: These, effectué au Département Minéralurgic du Bureau de Recherche Géologiquc et Miniéres á Orléans, France, Mars 1984.
Fletcher, P. and Sposito, G., Clay Miner., 1989, vol. 24, p. 375.
Bergaya, F., Stoiazzo, J.P., Trauth, N., and Van Damme, H., Clay Miner., 1986, vol. 21, p. 965.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2007, Vol. 43, No. 2, pp. 175–187.
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Kriaa, A., Hamdi, N. & Srasra, E. Acid-base chemistry of montmorillonitic and beidellitic-montmorillonitic smectite. Russ J Electrochem 43, 167–177 (2007). https://doi.org/10.1134/S102319350702005X
Received:
Issue Date:
DOI: https://doi.org/10.1134/S102319350702005X