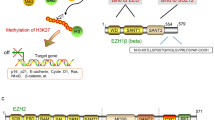
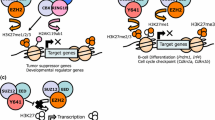
Abstract
PRC2 (Polycomb repressive complex 2) is a conserved protein complex in multicellular organisms that is required to maintain gene repression. The catalytic subunit of PRC2, the EZH2 protein, provides the methylation of histone H3K27 (H3K27me1/2/3). It was demonstrated that a number of human cancers were associated with overexpression of PRC2 subunits, as well as with mutations that enhanced the EZH2 catalytic activity. At the same time, a group of cancers correlate with mutations that inhibit PRC2. A number of small molecule inhibitors to the PRC2 subunits have been developed, primarily to EZH2. One of these, tazemetostat, received approval in January 2020 in the United States for the treatment of epithelioid sarcoma. This review focuses on the role of PRC2 in cancer development and summarizes information on the designed PRC2 inhibitors.
Similar content being viewed by others
REFERENCES
Bracken, A.P., Brien, G.L., and Verrijzer, C.P., Dangerous liaisons: interplay between SWI/SNF, NuRD, and Polycomb in chromatin regulation and cancer, Genes Dev., 2019, vol. 33, nos. 15—16, pp. 936—959. https://doi.org/10.1101/gad.326066.119
Grossniklaus, U. and Paro, R., Transcriptional silencing by polycomb-group proteins, Cold Spring Harb. Perspect. Biol., 2014, vol. 6, no. 11. a019331. https://doi.org/10.1101/cshperspect.a019331
Kuroda, M.I., Kang, H., De, S., and Kassis, J.A., Dynamic competition of polycomb and trithorax in transcriptional programming, Annu. Rev. Biochem., 2020, vol. 89, pp. 235—253. https://doi.org/10.1146/annurev-biochem-120219-103641
Piunti, A. and Shilatifard, A., Epigenetic balance of gene expression by Polycomb and COMPASS families, Science, 2016, vol. 352, no. 6290. aad9780. https://doi.org/10.1126/science.aad9780
Schuettengruber, B., Bourbon, H.M., Di Croce, L., and Cavalli, G., Genome regulation by polycomb and trithorax: 70 years and counting, Cell, 2017, vol. 171, no. 1, pp. 34—57. https://doi.org/10.1016/j.cell.2017.08.002
Chetverina, D.A., Elizar’ev, P.V., Lomaev, D.V., et al., Control of the gene activity by polycomb and trithorax group proteins in Drosophila, Genetika, 2017, vol. 53, no. 2, pp. 133—154.
Erokhin, M., Georgiev, P., and Chetverina, D., Drosophila DNA-binding proteins in polycomb repression, Epigenomes, 2018, vol. 2, no. 1, p. 1. https://doi.org/10.3390/epigenomes2010001
Kassis, J.A., Kennison, J.A., and Tamkun, J.W., Polycomb and trithorax group genes in Drosophila, Genetics, 2017, vol. 206, no. 4, pp. 1699—1725. https://doi.org/10.1534/genetics.115.185116
Mozgova, I. and Hennig, L., The polycomb group protein regulatory network, Annu. Rev. Plant Biol., 2015, vol. 66, pp. 269—296. https://doi.org/10.1146/annurev-arplant-043014-115627
Deevy, O. and Bracken, A.P., PRC2 functions in development and congenital disorders, Development, 2019, vol. 146, no. 19. https://doi.org/10.1242/dev.181354
Kouznetsova, V.L., Tchekanov, A., Li, X., et al., Polycomb repressive 2 complex—molecular mechanisms of function, Protein Sci., 2019, vol. 28, no. 8, pp. 1387—1399. https://doi.org/10.1002/pro.3647
Yu, J.R., Lee, C.H., Oksuz, O., et al., PRC2 is high maintenance, Genes Dev., 2019, vol. 33, nos. 15—16, pp. 903—935. https://doi.org/10.1101/gad.325050.119
Czermin, B., Melfi, R., McCabe, D., et al., Drosophila enhancer of zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites, Cell, 2002, vol. 111, no. 2, pp. 185—196.
Muller, J., Hart, C.M., Francis, N.J., et al., Histone methyltransferase activity of a Drosophila Polycomb group repressor complex, Cell, 2002, vol. 111, no. 2, pp. 197—208.
Cao, R., Wang, L., Wang, H., et al., Role of histone H3 lysine 27 methylation in Polycomb-group silencing, Science, 2002, vol. 298, no. 5595, pp. 1039—1043. https://doi.org/10.1126/science.1076997
Ferrari, K.J., Scelfo, A., Jammula, S., et al., Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity, Mol. Cell, 2014, vol. 53, no. 1, pp. 49—62. https://doi.org/10.1016/j.molcel.2013.10.030
Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., et al., Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of zeste protein, Genes Dev., 2002, vol. 16, no. 22, pp. 2893—2905. https://doi.org/10.1101/gad.1035902
Cao, R. and Zhang, Y., SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex, Mol. Cell, 2004, vol. 15, no. 1, pp. 57—67. https://doi.org/10.1016/j.molcel.2004.06.020
Montgomery, N.D., Yee, D., Chen, A., et al., The murine polycomb group protein Eed is required for global histone H3 lysine-27 methylation, Curr. Biol., 2005, vol. 15, no. 10, pp. 942—947. https://doi.org/10.1016/j.cub.2005.04.051
Pasini, D., Bracken, A.P., Jensen, M.R., et al., Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity, EMBO J., 2004, vol. 23, no. 20, pp. 4061—4071. https://doi.org/10.1038/sj.emboj.7600402
Margueron, R., Justin, N., Ohno, K., et al., Role of the polycomb protein EED in the propagation of repressive histone marks, Nature, 2009, vol. 461, no. 7265, pp. 762—767. https://doi.org/10.1038/nature08398
Faust, C., Schumacher, A., Holdener, B., and Magnuson, T., The eed mutation disrupts anterior mesoderm production in mice, Development, 1995, vol. 121, no. 2, pp. 273—285.
O’Carroll, D., Erhardt, S., Pagani, M., et al., The polycomb-group gene Ezh2 is required for early mouse development, Mol. Cell Biol., 2001, vol. 21, no. 13, pp. 4330—4336. https://doi.org/10.1128/MCB.21.13.4330-4336.2001
Margueron, R., Li, G., Sarma, K., et al., Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms, Mol. Cell, 2008, vol. 32, no. 4, pp. 503—518. https://doi.org/10.1016/j.molcel.2008.11.004
Ezhkova, E., Lien, W.H., Stokes, N., et al., EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair, Genes Dev., 2011, vol. 25, no. 5, pp. 485—498. https://doi.org/10.1101/gad.2019811
Son, J., Shen, S.S., Margueron, R., and Reinberg, D., Nucleosome-binding activities within JARID2 and EZH1 regulate the function of PRC2 on chromatin, Genes Dev., 2013, vol. 27, no. 24, pp. 2663—2677. https://doi.org/10.1101/gad.225888.113
Shen, X., Liu, Y., Hsu, Y.J., et al., EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency, Mol. Cell, 2008, vol. 32, no. 4, pp. 491—502. https://doi.org/10.1016/j.molcel.2008.10.016
Genta, S., Pirosa, M.C., and Stathis, A., BET and EZH2 inhibitors: novel approaches for targeting cancer, Curr. Oncol. Rep., 2019, vol. 21, no. 2, p. 13. https://doi.org/10.1007/s11912-019-0762-x
Richart, L. and Margueron, R., Drugging histone methyltransferases in cancer, Curr. Opin. Chem. Biol., 2020, vol. 56, pp. 51—62. https://doi.org/10.1016/j.cbpa.2019.11.009
Hoy, S.M., Tazemetostat: first approval, Drugs, 2020, vol. 80, no. 5, pp. 513—521. https://doi.org/10.1007/s40265-020-01288-x
Italiano, A., Targeting epigenetics in sarcomas through EZH2 inhibition, J. Hematol. Oncol., 2020, vol. 13, no. 1, p. 33. https://doi.org/10.1186/s13045-020-00868-4
Rothbart, S.B. and Baylin, S.B., Epigenetic therapy for epithelioid sarcoma, Cell, 2020, vol. 181, no. 2, p. 211. https://doi.org/10.1016/j.cell.2020.03.042
Comet, I., Riising, E.M., Leblanc, B., and Helin, K., Maintaining cell identity: PRC2-mediated regulation of transcription and cancer, Nat. Rev. Cancer, 2016, vol. 16, no. 12, pp. 803—810. https://doi.org/10.1038/nrc.2016.83
Kim, K.H. and Roberts, C.W., Targeting EZH2 in cancer, Nat. Med., 2016, vol. 22, no. 2, pp. 128—134. https://doi.org/10.1038/nm.4036
Lue, J.K. and Amengual, J.E., Emerging EZH2 inhibitors and their application in lymphoma, Curr. Hematol. Malig. Rep., 2018, vol. 13, no. 5, pp. 369—382. https://doi.org/10.1007/s11899-018-0466-6
Yamagishi, M. and Uchimaru, K., Targeting EZH2 in cancer therapy, Curr. Opin. Oncol., 2017, vol. 29, no. 5, pp. 375—381. https://doi.org/10.1097/CCO.0000000000000390
Bracken, A.P., Pasini, D., Capra, M., et al., EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer, EMBO J., 2003, vol. 22, no. 20, pp. 5323—5335. https://doi.org/10.1093/emboj/cdg542
Lee, S.R., Roh, Y.G., Kim, S.K., et al., Activation of EZH2 and SUZ12 regulated by E2F1 predicts the disease progression and aggressive characteristics of bladder cancer, Clin. Cancer Res., 2015, vol. 21, no. 23, pp. 5391—5403. https://doi.org/10.1158/1078-0432.CCR-14-2680
Takawa, M., Masuda, K., Kunizaki, M., et al., Validation of the histone methyltransferase EZH2 as a therapeutic target for various types of human cancer and as a prognostic marker, Cancer Sci., 2011, vol. 102, no. 7, pp. 1298—1305. https://doi.org/10.1111/j.1349-7006.2011.01958.x
Okosun, J., Bodor, C., Wang, J., et al., Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma, Nat. Genet., 2014, vol. 46, no. 2, pp. 176—181. https://doi.org/10.1038/ng.2856
Kienle, D., Katzenberger, T., Ott, G., et al., Quantitative gene expression deregulation in mantle-cell lymphoma: correlation with clinical and biologic factors, J. Clin. Oncol., 2007, vol. 25, no. 19, pp. 2770—2777. https://doi.org/10.1200/JCO.2006.08.7999
Lin, Y.L., Zou, Z.K., Su, H.Y., and Huang, Y.Q., Expression of MiR101 and EZH2 in patients with mantle cell lymphoma and its clinical significance, Zhongguo Shi Yan Xue Ye Xue Za Zhi, 2019, vol. 27, no. 3, pp. 820—826. https://doi.org/10.19746/j.cnki.issn.1009-2137.2019.03.029
Yan, J., Ng, S.B., Tay, J.L., et al., EZH2 overexpression in natural killer/T-cell lymphoma confers growth advantage independently of histone methyltransferase activity, Blood, 2013, vol. 121, no. 22, pp. 4512—4520. https://doi.org/10.1182/blood-2012-08-450494
Pawlyn, C., Bright, M.D., Buros, A.F., et al., Overexpression of EZH2 in multiple myeloma is associated with poor prognosis and dysregulation of cell cycle control, Blood Cancer J., 2017, vol. 7, no. 3. e549. https://doi.org/10.1038/bcj.2017.27
Wilson, B.G., Wang, X., Shen, X., et al., Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation, Cancer Cell, 2010, vol. 18, no. 4, pp. 316—328. https://doi.org/10.1016/j.ccr.2010.09.006
Bachmann, I.M., Halvorsen, O.J., Collett, K., et al., EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast, J. Clin. Oncol., 2006, vol. 24, no. 2, pp. 268—273. https://doi.org/10.1200/JCO.2005.01.5180
Collett, K., Eide, G.E., Arnes, J., et al., Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer, Clin. Cancer Res., 2006, vol. 12, no. 4, pp. 1168—1174. https://doi.org/10.1158/1078-0432.CCR-05-1533
Gonzalez, M.E., Moore, H.M., Li, X., et al., EZH2 expands breast stem cells through activation of NOTCH1 signaling, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, no. 8, pp. 3098—3103. https://doi.org/10.1073/pnas.1308953111
Kleer, C.G., Cao, Q., Varambally, S., et al., EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells, Proc. Natl. Acad. Sci. U.S.A., 2003, vol. 100, no. 20, pp. 11606—11611. https://doi.org/10.1073/pnas.1933744100
Pietersen, A.M., Horlings, H.M., Hauptmann, M., et al., EZH2 and BMI1 inversely correlate with prognosis and TP53 mutation in breast cancer, Breast Cancer Res., 2008, vol. 10, no. 6, p. R109. https://doi.org/10.1186/bcr2214
Puppe, J., Drost, R., Liu, X., et al., BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb Repressive Complex 2-inhibitor 3-deazaneplanocin A, Breast Cancer Res., 2009, vol. 11, no. 4, p. R63. https://doi.org/10.1186/bcr2354
Yu, H., Simons, D.L., Segall, I., et al., PRC2/EED-EZH2 complex is up-regulated in breast cancer lymph node metastasis compared to primary tumor and correlates with tumor proliferation in situ, PLoS One, 2012, vol. 7, no. 12. e51239. https://doi.org/10.1371/journal.pone.0051239
Liu, Y.L., Gao, X., Jiang, Y., et al., Expression and clinicopathological significance of EED, SUZ12 and EZH2 mRNA in colorectal cancer, J. Cancer Res. Clin. Oncol., 2015, vol. 141, no. 4, pp. 661—669. https://doi.org/10.1007/s00432-014-1854-5
Ohuchi, M., Sakamoto, Y., Tokunaga, R., et al., Increased EZH2 expression during the adenoma—carcinoma sequence in colorectal cancer, Oncol. Lett., 2018, vol. 16, no. 4, pp. 5275—5281. https://doi.org/10.3892/ol.2018.9240
Wang, C.G., Ye, Y.J., Yuan, J., et al., EZH2 and STAT6 expression profiles are correlated with colorectal cancer stage and prognosis, World J. Gastroenterol., 2010, vol. 16, no. 19, pp. 2421—2427. https://doi.org/10.3748/wjg.v16.i19.2421
He, L.J., Cai, M.Y., Xu, G.L., et al., Prognostic significance of overexpression of EZH2 and H3k27me3 proteins in gastric cancer, Asian Pac. J. Cancer Prev., 2012, vol. 13, no. 7, pp. 3173—3178. https://doi.org/10.7314/apjcp.2012.13.7.3173
Pan, Y.M., Wang, C.G., Zhu, M., et al., STAT3 signaling drives EZH2 transcriptional activation and mediates poor prognosis in gastric cancer, Mol. Cancer, 2016, vol. 15, no. 1, p. 79. https://doi.org/10.1186/s12943-016-0561-z
Lei, Q., Shen, F., Wu, J., et al., MiR-101, downregulated in retinoblastoma, functions as a tumor suppressor in human retinoblastoma cells by targeting EZH2, Oncol. Rep., 2014, vol. 32, no. 1, pp. 261—269. https://doi.org/10.3892/or.2014.3167
Wagener, N., Macher-Goeppinger, S., Pritsch, M., et al., Enhancer of zeste homolog 2 (EZH2) expression is an independent prognostic factor in renal cell carcinoma, BMC Cancer, 2010, vol. 10, p. 524. https://doi.org/10.1186/1471-2407-10-524
Zhang, M.J., Chen, D.S., Li, H., et al., Clinical significance of USP7 and EZH2 in predicting prognosis of laryngeal squamous cell carcinoma and their possible functional mechanism, Int. J. Clin. Exp. Pathol., 2019, vol. 12, no. 6, pp. 2184—2194.
Sudo, T., Utsunomiya, T., Mimori, K., et al., Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma, Br. J. Cancer, 2005, vol. 92, no. 9, pp. 1754—1758. https://doi.org/10.1038/sj.bjc.6602531
Nakagawa, S., Okabe, H., Sakamoto, Y., et al., Enhancer of zeste homolog 2 (EZH2) promotes progression of cholangiocarcinoma cells by regulating cell cycle and apoptosis, Ann. Surg. Oncol., 2013, vol. 20, suppl 3, pp. S667—S675. https://doi.org/10.1245/s10434-013-3135-y
Cao, W., Ribeiro, RdeO., Liu, D., et al., EZH2 promotes malignant behaviors via cell cycle dysregulation and its mRNA level associates with prognosis of patient with non-small cell lung cancer, PLoS One, 2012, vol. 7, no. 12. e52984. https://doi.org/10.1371/journal.pone.0052984
Kikuchi, J., Kinoshita, I., Shimizu, Y., et al., Distinctive expression of the polycomb group proteins Bmi1 polycomb ring finger oncogene and enhancer of zeste homolog 2 in nonsmall cell lung cancers and their clinical and clinicopathologic significance, Cancer, 2010, vol. 116, no. 12, pp. 3015—3024. https://doi.org/10.1002/cncr.25128
Liu, H., Li, W., Yu, X., et al., EZH2-mediated Puma gene repression regulates non-small cell lung cancer cell proliferation and cisplatin-induced apoptosis, Oncotarget, 2016, vol. 7, no. 35, pp. 56338—56354. https://doi.org/10.18632/oncotarget.10841
Ciarapica, R., Russo, G., Verginelli, F., et al., Deregulated expression of miR-26a and Ezh2 in rhabdomyosarcoma, Cell Cycle, 2009, vol. 8, no. 1, pp. 172—175. https://doi.org/10.4161/cc.8.1.7292
Li, H., Cai, Q., Godwin, A.K., and Zhang, R., Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells, Mol. Cancer Res., 2010, vol. 8, no. 12, pp. 1610—1618. https://doi.org/10.1158/1541-7786.MCR-10-0398
Li, H., Cai, Q., Wu, H., et al., SUZ12 promotes human epithelial ovarian cancer by suppressing apoptosis via silencing HRK, Mol. Cancer Res., 2012, vol. 10, no. 11, pp. 1462—1472. https://doi.org/10.1158/1541-7786.MCR-12-0335
Lu, C., Han, H.D., Mangala, L.S., et al., Regulation of tumor angiogenesis by EZH2, Cancer Cell, 2010, vol. 18, no. 2, pp. 185—197. https://doi.org/10.1016/j.ccr.2010.06.016
Crea, F., Hurt, E.M., Mathews, L.A., et al., Pharmacologic disruption of Polycomb Repressive Complex 2 inhibits tumorigenicity and tumor progression in prostate cancer, Mol. Cancer, 2011, vol. 10, p. 40. https://doi.org/10.1186/1476-4598-10-40
Varambally, S., Dhanasekaran, S.M., Zhou, M., et al., The polycomb group protein EZH2 is involved in progression of prostate cancer, Nature, 2002, vol. 419, no. 6907, pp. 624—629. https://doi.org/10.1038/nature01075
Saramaki, O.R., Tammela, T.L., Martikainen, P.M., et al., The gene for polycomb group protein enhancer of zeste homolog 2 (EZH2) is amplified in late-stage prostate cancer, Genes Chromosomes Cancer, 2006, vol. 45, no. 7, pp. 639—645. https://doi.org/10.1002/gcc.20327
Borbone, E., Troncone, G., Ferraro, A., et al., Enhancer of zeste homolog 2 overexpression has a role in the development of anaplastic thyroid carcinomas, J. Clin. Endocrinol. Metab., 2011, vol. 96, no. 4, pp. 1029—1038. https://doi.org/10.1210/jc.2010-1784
Masudo, K., Suganuma, N., Nakayama, H., et al., EZH2 overexpression as a useful prognostic marker for aggressive behaviour in thyroid cancer, In Vivo, 2018, vol. 32, no. 1, pp. 25—31. https://doi.org/10.21873/invivo.11200
Azizmohammadi, S., Azizmohammadi, S., Safari, A., et al., High-level expression of RIPK4 and EZH2 contributes to lymph node metastasis and predicts favorable prognosis in patients with cervical cancer, Oncol. Res., 2017, vol. 25, no. 4, pp. 495—501. https://doi.org/10.3727/096504016X14749735594687
Jia, N., Li, Q., Tao, X., et al., Enhancer of zeste homolog 2 is involved in the proliferation of endometrial carcinoma, Oncol. Lett., 2014, vol. 8, no. 5, pp. 2049—2054. https://doi.org/10.3892/ol.2014.2437
Abudurexiti, M., Xie, H., Jia, Z., et al., Development and external validation of a novel 12-gene signature for prediction of overall survival in muscle-invasive bladder cancer, Front. Oncol., 2019, vol. 9, p. 856. https://doi.org/10.3389/fonc.2019.00856
Martin-Perez, D., Sanchez, E., Maestre, L., et al., Deregulated expression of the polycomb-group protein SUZ12 target genes characterizes mantle cell lymphoma, Am. J. Pathol., 2010, vol. 177, no. 2, pp. 930—942. https://doi.org/10.2353/ajpath.2010.090769
Iliopoulos, D., Lindahl-Allen, M., Polytarchou, C., et al., Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells, Mol. Cell, 2010, vol. 39, no. 5, pp. 761—772. https://doi.org/10.1016/j.molcel.2010.08.013
Xia, R., Jin, F.Y., Lu, K., et al., SUZ12 promotes gastric cancer cell proliferation and metastasis by regulating KLF2 and E-cadherin, Tumour Biol., 2015, vol. 36, no. 7, pp. 5341—5351. https://doi.org/10.1007/s13277-015-3195-7
Liu, C., Shi, X., Wang, L., et al., SUZ12 is involved in progression of non-small cell lung cancer by promoting cell proliferation and metastasis, Tumour Biol., 2014, vol. 35, no. 6, pp. 6073—6082. https://doi.org/10.1007/s13277-014-1804-5
Bodor, C., Grossmann, V., Popov, N., et al., EZH2 mutations are frequent and represent an early event in follicular lymphoma, Blood, 2013, vol. 122, no. 18, pp. 3165—3168. https://doi.org/10.1182/blood-2013-04-496893
Bodor, C., O’Riain, C., Wrench, D., et al., EZH2 Y641 mutations in follicular lymphoma, Leukemia, 2011, vol. 25, no. 4, pp. 726—729. https://doi.org/10.1038/leu.2010.311
Lohr, J.G., Stojanov, P., Lawrence, M.S., et al., Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing, Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 10, pp. 3879—3884. https://doi.org/10.1073/pnas.1121343109
Majer, C.R., Jin, L., Scott, M.P., et al., A687V EZH2 is a gain-of-function mutation found in lymphoma patients, FEBS Lett., 2012, vol. 586, no. 19, pp. 3448—3451. https://doi.org/10.1016/j.febslet.2012.07.066
Morin, R.D., Johnson, N.A., Severson, T.M., et al., Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin, Nat. Genet., 2010, vol. 42, no. 2, pp. 181—185. https://doi.org/10.1038/ng.518
Morin, R.D., Mendez-Lago, M., Mungall, A.J., et al., Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma, Nature, 2011, vol. 476, no. 7360, pp. 298—303. https://doi.org/10.1038/nature10351
Reddy, A., Zhang, J., Davis, N.S., et al., Genetic and functional drivers of diffuse large B cell lymphoma, Cell, 2017, vol. 171, no. 2, pp. 481—494. e415. https://doi.org/10.1016/j.cell.2017.09.027
Ryan, R.J., Nitta, M., Borger, D., et al., EZH2 codon 641 mutations are common in BCL2-rearranged germinal center B cell lymphomas, PLoS One, 2011, vol. 6, no. 12. e28585. https://doi.org/10.1371/journal.pone.0028585
Calebiro, D., Grassi, E.S., Eszlinger, M., et al., Recurrent EZH1 mutations are a second hit in autonomous thyroid adenomas, J. Clin. Invest., 2016, vol. 126, no. 9, pp. 3383—3388. https://doi.org/10.1172/JCI84894
Ntziachristos, P., Tsirigos, A., Van Vlierberghe, P., et al., Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia, Nat. Med., 2012, vol. 18, no. 2, pp. 298—301. https://doi.org/10.1038/nm.2651
Zhang, J., Ding, L., Holmfeldt, L., et al., The genetic basis of early T-cell precursor acute lymphoblastic leukaemia, Nature, 2012, vol. 481, no. 7380, pp. 157—163. https://doi.org/10.1038/nature10725
Puda, A., Milosevic, J.D., Berg, T., et al., Frequent deletions of JARID2 in leukemic transformation of chronic myeloid malignancies, Am. J. Hematol., 2012, vol. 87, no. 3, pp. 245—250. https://doi.org/10.1002/ajh.22257
Bejar, R., Stevenson, K., Abdel-Wahab, O., et al., Clinical effect of point mutations in myelodysplastic syndromes, N. Engl. J. Med., 2011, vol. 364, no. 26, pp. 2496—2506. https://doi.org/10.1056/NEJMoa1013343
Ernst, T., Chase, A.J., Score, J., et al., Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders, Nat. Genet., 2010, vol. 42, no. 8, pp. 722—726. https://doi.org/10.1038/ng.621
Guglielmelli, P., Biamonte, F., Score, J., et al., EZH2 mutational status predicts poor survival in myelofibrosis, Blood, 2011, vol. 118, no. 19, pp. 5227—5234. https://doi.org/10.1182/blood-2011-06-363424
Khan, S.N., Jankowska, A.M., Mahfouz, R., et al., Multiple mechanisms deregulate EZH2 and histone H3 lysine 27 epigenetic changes in myeloid malignancies, Leukemia, 2013, vol. 27, no. 6, pp. 1301—1309. https://doi.org/10.1038/leu.2013.80
Nikoloski, G., Langemeijer, S.M., Kuiper, R.P., et al., Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes, Nat. Genet., 2010, vol. 42, no. 8, pp. 665—667. https://doi.org/10.1038/ng.620
Zhang, Q., Han, Q., Zi, J., et al., Mutations in EZH2 are associated with poor prognosis for patients with myeloid neoplasms, Genes Dis., 2019, vol. 6, no. 3, pp. 276—281. https://doi.org/10.1016/j.gendis.2019.05.001
Score, J., Hidalgo-Curtis, C., Jones, A.V., et al., Inactivation of polycomb repressive complex 2 components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms, Blood, 2012, vol. 119, no. 5, pp. 1208—1213. https://doi.org/10.1182/blood-2011-07-367243
De Raedt, T., Beert, E., Pasmant, E., et al., PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies, Nature, 2014, vol. 514, no. 7521, pp. 247—251. https://doi.org/10.1038/nature13561
Lee, W., Teckie, S., Wiesner, T., et al., PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors, Nat. Genet., 2014, vol. 46, no. 11, pp. 1227—1232. https://doi.org/10.1038/ng.3095
Zhang, M., Wang, Y., Jones, S., et al., Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors, Nat. Genet., 2014, vol. 46, no. 11, pp. 1170—1172. https://doi.org/10.1038/ng.3116
Koontz, J.I., Soreng, A.L., Nucci, M., et al., Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors, Proc. Natl. Acad. Sci. U.S.A., 2001, vol. 98, no. 11, pp. 6348—6353. https://doi.org/10.1073/pnas.101132598
Li, H., Ma, X., Wang, J., et al., Effects of rearrangement and allelic exclusion of JJAZ1/SUZ12 on cell proliferation and survival, Proc. Natl. Acad. Sci. U.S.A., 2007, vol. 104, no. 50, pp. 20001—20006. https://doi.org/10.1073/pnas.0709986104
Ma, X., Wang, J., Wang, J., et al., The JAZF1-SUZ12 fusion protein disrupts PRC2 complexes and impairs chromatin repression during human endometrial stromal tumorogenesis, Oncotarget, 2017, vol. 8, no. 3, pp. 4062—4078. https://doi.org/10.18632/oncotarget.13270
Makise, N., Sekimizu, M., Kobayashi, E., et al., Low-grade endometrial stromal sarcoma with a novel MEAF6-SUZ12 fusion, Virchows Arch., 2019, vol. 475, no. 4, pp. 527—531. https://doi.org/10.1007/s00428-019-02588-8
Ueda, T., Sanada, M., Matsui, H., et al., EED mutants impair polycomb repressive complex 2 in myelodysplastic syndrome and related neoplasms, Leukemia, 2012, vol. 26, no. 12, pp. 2557—2560. https://doi.org/10.1038/leu.2012.146
Boileau, M., Shirinian, M., Gayden, T., et al., Mutant H3 histones drive human pre-leukemic hematopoietic stem cell expansion and promote leukemic aggressiveness, Nat. Commun., 2019, vol. 10, no. 1, p. 2891. https://doi.org/10.1038/s41467-019-10705-z
Schwartzentruber, J., Korshunov, A., Liu, X.Y., et al., Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma, Nature, 2012, vol. 482, no. 7384, pp. 226—231. https://doi.org/10.1038/nature10833
Sturm, D., Witt, H., Hovestadt, V., et al., Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma, Cancer Cell, 2012, vol. 22, no. 4, pp. 425—437. https://doi.org/10.1016/j.ccr.2012.08.024
Wu, G., Broniscer, A., McEachron, T.A., et al., Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas, Nat. Genet., 2012, vol. 44, no. 3, pp. 251—253. https://doi.org/10.1038/ng.1102
McCabe, M.T., Graves, A.P., Ganji, G., et al., Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27), Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 8, pp. 2989—2994. https://doi.org/10.1073/pnas.1116418109
Ott, H.M., Graves, A.P., Pappalardi, M.B., et al., A687V EZH2 is a driver of histone H3 lysine 27 (H3K27) hypertrimethylation, Mol. Cancer Ther., 2014, vol. 13, no. 12, pp. 3062—3073. https://doi.org/10.1158/1535-7163.MCT-13-0876
Sneeringer, C.J., Scott, M.P., Kuntz, K.W., et al., Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas, Proc. Natl. Acad. Sci. U.S.A., 2010, vol. 107, no. 49, pp. 20980—20985. https://doi.org/10.1073/pnas.1012525107
Yap, D.B., Chu, J., Berg, T., et al., Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation, Blood, 2011, vol. 117, no. 8, pp. 2451—2459. https://doi.org/10.1182/blood-2010-11-321208
Beguelin, W., Popovic, R., Teater, M., et al., EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation, Cancer Cell, 2013, vol. 23, no. 5, pp. 677—692. https://doi.org/10.1016/j.ccr.2013.04.011
Chang, C.J., Yang, J.Y., Xia, W., et al., EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling, Cancer Cell, 2011, vol. 19, no. 1, pp. 86—100. https://doi.org/10.1016/j.ccr.2010.10.035
Herrera-Merchan, A., Arranz, L., Ligos, J.M., et al., Ectopic expression of the histone methyltransferase Ezh2 in haematopoietic stem cells causes myeloproliferative disease, Nat. Commun., 2012, vol. 3, p. 623. https://doi.org/10.1038/ncomms1623
Min, J., Zaslavsky, A., Fedele, G., et al., An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB, Nat. Med., 2010, vol. 16, no. 3, pp. 286—294. https://doi.org/10.1038/nm.2100
Berg, T., Thoene, S., Yap, D., et al., A transgenic mouse model demonstrating the oncogenic role of mutations in the polycomb-group gene EZH2 in lymphomagenesis, Blood, 2014, vol. 123, no. 25, pp. 3914—3924. https://doi.org/10.1182/blood-2012-12-473439
Amatangelo, M.D., Garipov, A., Li, H., et al., Three-dimensional culture sensitizes epithelial ovarian cancer cells to EZH2 methyltransferase inhibition, Cell Cycle, 2013, vol. 12, no. 13, pp. 2113—2119. https://doi.org/10.4161/cc.25163
Kim, W., Bird, G.H., Neff, T., et al., Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer, Nat. Chem. Biol., 2013, vol. 9, no. 10, pp. 643—650. https://doi.org/10.1038/nchembio.1331
Knutson, S.K., Warholic, N.M., Wigle, T.J., et al., Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2, Proc. Natl. Acad. Sci. U.S.A., 2013, vol. 110, no. 19, pp. 7922—7927. https://doi.org/10.1073/pnas.1303800110
Neff, T., Sinha, A.U., Kluk, M.J., et al., Polycomb repressive complex 2 is required for MLL-AF9 leukemia, Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 13, pp. 5028—5033. https://doi.org/10.1073/pnas.1202258109
Shi, J., Wang, E., Zuber, J., et al., The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9;Nras(G12D) acute myeloid leukemia, Oncogene, 2013, vol. 32, no. 7, pp. 930—938. https://doi.org/10.1038/onc.2012.110
Tanaka, S., Miyagi, S., Sashida, G., et al., Ezh2 augments leukemogenicity by reinforcing differentiation blockage in acute myeloid leukemia, Blood, 2012, vol. 120, no. 5, pp. 1107—1117. https://doi.org/10.1182/blood-2011-11-394932
Bender, S., Tang, Y., Lindroth, A.M., et al., Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas, Cancer Cell, 2013, vol. 24, no. 5, pp. 660—672. https://doi.org/10.1016/j.ccr.2013.10.006
Chan, K.M., Fang, D., Gan, H., et al., The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression, Genes Dev., 2013, vol. 27, no. 9, pp. 985—990. https://doi.org/10.1101/gad.217778.113
Justin, N., Zhang, Y., Tarricone, C., et al., Structural basis of oncogenic histone H3K27M inhibition of human polycomb repressive complex 2, Nat. Commun., 2016, vol. 7, p. 11316. https://doi.org/10.1038/ncomms11316
Lewis, P.W., Muller, M.M., Koletsky, M.S., et al., Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma, Science, 2013, vol. 340, no. 6134, pp. 857—861. https://doi.org/10.1126/science.1232245
Lee, C.H., Yu, J.R., Granat, J., et al., Automethylation of PRC2 promotes H3K27 methylation and is impaired in H3K27M pediatric glioma, Genes Dev., 2019, vol. 33, nos. 19–20, pp. 1428—1440. https://doi.org/10.1101/gad.328773.119
Hubner, J.M., Muller, T., Papageorgiou, D.N., et al., EZHIP/CXorf67 mimics K27M mutated oncohistones and functions as an intrinsic inhibitor of PRC2 function in aggressive posterior fossa ependymoma, Neuro. Oncol., 2019, vol. 21, no. 7, pp. 878—889. https://doi.org/10.1093/neuonc/noz058
Jain, S.U., Do, T.J., Lund, P.J., et al., PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism, Nat. Commun., 2019, vol. 10, no. 1, p. 2146. https://doi.org/10.1038/s41467-019-09981-6
Piunti, A., Smith, E.R., Morgan, M.A.J., et al., CATACOMB: an endogenous inducible gene that antagonizes H3K27 methylation activity of Polycomb repressive complex 2 via an H3K27M-like mechanism, Sci. Adv., 2019, vol. 5, no. 7, p. eaax2887. https://doi.org/10.1126/sciadv.aax2887
Ragazzini, R., Perez-Palacios, R., Baymaz, I.H., et al., EZHIP constrains Polycomb Repressive Complex 2 activity in germ cells, Nat. Commun., 2019, vol. 10, no. 1, p. 3858. https://doi.org/10.1038/s41467-019-11800-x
Abdel-Wahab, O. and Dey, A., The ASXL-BAP1 axis: new factors in myelopoiesis, cancer and epigenetics, Leukemia, 2013, vol. 27, no. 1, pp. 10—15. https://doi.org/10.1038/leu.2012.288
Danis, E., Yamauchi, T., Echanique, K., et al., Ezh2 controls an early hematopoietic program and growth and survival signaling in early T cell precursor acute lymphoblastic leukemia, Cell Rep., 2016, vol. 14, no. 8, pp. 1953—1965. https://doi.org/10.1016/j.celrep.2016.01.064
Booth, C.A.G., Barkas, N., Neo, W.H., et al., Ezh2 and Runx1 mutations collaborate to initiate lympho-myeloid leukemia in early thymic progenitors, Cancer Cell, 2018, vol. 33, no. 2, pp. 274—291. e278. https://doi.org/10.1016/j.ccell.2018.01.006
Wang, C., Oshima, M., Sato, D., et al., Ezh2 loss propagates hypermethylation at T cell differentiation-regulating genes to promote leukemic transformation, J. Clin. Invest., 2018, vol. 128, no. 9, pp. 3872—3886. https://doi.org/10.1172/JCI94645
Broux, M., Prieto, C., Demeyer, S., et al., Suz12 inactivation cooperates with JAK3 mutant signaling in the development of T-cell acute lymphoblastic leukemia, Blood, 2019, vol. 134, no. 16, pp. 1323—1336. https://doi.org/10.1182/blood.2019000015
Abdel-Wahab, O., Adli, M., LaFave, L.M., et al., ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression, Cancer Cell, 2012, vol. 22, no. 2, pp. 180—193. https://doi.org/10.1016/j.ccr.2012.06.032
Lane, A.A., Chapuy, B., Lin, C.Y., et al., Triplication of a 21q22 region contributes to B cell transformation through HMGN1 overexpression and loss of histone H3 Lys27 trimethylation, Nat. Genet., 2014, vol. 46, no. 6, pp. 618—623. https://doi.org/10.1038/ng.2949
Sashida, G., Harada, H., Matsui, H., et al., Ezh2 loss promotes development of myelodysplastic syndrome but attenuates its predisposition to leukaemic transformation, Nat. Commun., 2014, vol. 5, p. 4177. https://doi.org/10.1038/ncomms5177
Maertens, O. and Cichowski, K., An expanding role for RAS GTPase activating proteins (RAS GAPs) in cancer, Adv. Biol. Regul., 2014, vol. 55, pp. 1—14. https://doi.org/10.1016/j.jbior.2014.04.002
Simon, C., Chagraoui, J., Krosl, J., et al., A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia, Genes Dev., 2012, vol. 26, no. 7, pp. 651—656. https://doi.org/10.1101/gad.186411.111
Souroullas, G.P., Jeck, W.R., Parker, J.S., et al., An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation, Nat. Med., 2016, vol. 22, no. 6, pp. 632—640. https://doi.org/10.1038/nm.4092
Tan, J., Yang, X., Zhuang, L., et al., Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells, Genes Dev., 2007, vol. 21, no. 9, pp. 1050—1063. https://doi.org/10.1101/gad.1524107
Miranda, T.B., Cortez, C.C., Yoo, C.B., et al., DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation, Mol. Cancer Ther., 2009, vol. 8, no. 6, pp. 1579—1588. https://doi.org/10.1158/1535-7163.MCT-09-0013
Knutson, S.K., Wigle, T.J., Warholic, N.M., et al., A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells, Nat. Chem. Biol., 2012, vol. 8, no. 11, pp. 890—896. https://doi.org/10.1038/nchembio.1084
McCabe, M.T., Ott, H.M., Ganji, G., et al., EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations, Nature, 2012, vol. 492, no. 7427, pp. 108—112. https://doi.org/10.1038/nature11606
Qi, W., Chan, H., Teng, L., et al., Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation, Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 52, pp. 21360—21365. https://doi.org/10.1073/pnas.1210371110
Knutson, S.K., Kawano, S., Minoshima, Y., et al., Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma, Mol. Cancer Ther., 2014, vol. 13, no. 4, pp. 842—854. https://doi.org/10.1158/1535-7163.MCT-13-0773
Konze, K.D., Ma, A., Li, F., et al., An orally bioavailable chemical probe of the lysine methyltransferases EZH2 and EZH1, ACS Chem. Biol., 2013, vol. 8, no. 6, pp. 1324—1334. https://doi.org/10.1021/cb400133j
Fujita, S., Honma, D., Adachi, N., et al., Dual inhibition of EZH1/2 breaks the quiescence of leukemia stem cells in acute myeloid leukemia, Leukemia, 2018, vol. 32, no. 4, pp. 855—864. https://doi.org/10.1038/leu.2017.300
Honma, D., Kanno, O., Watanabe, J., et al., Novel orally bioavailable EZH1/2 dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor, Cancer Sci., 2017, vol. 108, no. 10, pp. 2069—2078. https://doi.org/10.1111/cas.13326
He, Y., Selvaraju, S., Curtin, M.L., et al., The EED protein—protein interaction inhibitor A-395 inactivates the PRC2 complex, Nat. Chem. Biol., 2017, vol. 13, no. 4, pp. 389—395. https://doi.org/10.1038/nchembio.2306
Qi, W., Zhao, K., Gu, J., et al., An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED, Nat. Chem. Biol., 2017, vol. 13, no. 4, pp. 381—388. https://doi.org/10.1038/nchembio.2304
Ma, A., Stratikopoulos, E., Park, K.S., et al., Discovery of a first-in-class EZH2 selective degrader, Nat. Chem. Biol., 2020, vol. 16, no. 2, pp. 214—222. https://doi.org/10.1038/s41589-019-0421-4
Versteege, I., Sevenet, N., Lange, J., et al., Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer, Nature, 1998, vol. 394, no. 6689, pp. 203—206. https://doi.org/10.1038/28212
Kohashi, K. and Oda, Y., Oncogenic roles of SMARCB1/INI1 and its deficient tumors, Cancer Sci., 2017, vol. 108, no. 4, pp. 547—552. https://doi.org/10.1111/cas.13173
Jiao, L. and Liu, X., Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2, Science, 2015, vol. 350, no. 6258, p. aac4383. https://doi.org/10.1126/science.aac4383
Kasinath, V., Faini, M., Poepsel, S., et al., Structures of human PRC2 with its cofactors AEBP2 and JARID2, Science, 2018, vol. 359, no. 6378, pp. 940—944. https://doi.org/10.1126/science.aar5700
Khan, M., Walters, L.L., Li, Q., et al., Characterization and pharmacologic targeting of EZH2, a fetal retinal protein and epigenetic regulator, in human retinoblastoma, Lab. Invest., 2015, vol. 95, no. 11, pp. 1278—1290. https://doi.org/10.1038/labinvest.2015.104
Lai, A.C. and Crews, C.M., Induced protein degradation: an emerging drug discovery paradigm, Nat. Rev. Drug. Discov., 2017, vol. 16, no. 2, pp. 101—114. https://doi.org/10.1038/nrd.2016.211
Funding
This study was supported by the Russian Science Foundation (grant no. 18-74-10091).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Statement of compliance with standards of research involving humans as subjects. This article does not contain any research involving humans as a subject.
Additional information
Translated by N. Maleeva
Rights and permissions
About this article
Cite this article
Chetverina, D.A., Lomaev, D.V., Georgiev, P.G. et al. Genetic Impairments of PRC2 Activity in Oncology: Problems and Prospects. Russ J Genet 57, 258–272 (2021). https://doi.org/10.1134/S1022795421030042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795421030042