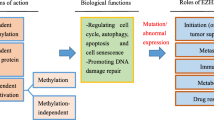
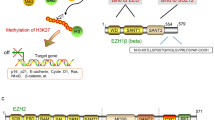
Abstract
Purpose of Review
Increasing evidence suggests that epigenome plays a central role in cancer development making it a promising target for anticancer treatments. Here, we review two new classes of epigenome-targeting agents: the bromodomain and extraterminal domain proteins (BET) inhibitors and the enhancer of zeste homolog (EZH2) inhibitors.
Recent Findings
Clinical research evaluating BET and EZH2 inhibitors is still at an early stage; however, both classes of drugs have demonstrated activity among different hematologic malignancies and solid tumors.
Summary
Several studies on BETi and EZH2i are ongoing to better define their potential role in cancer treatment, which patients are most likely to benefit and if the association with other drugs can improve their efficacy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Esteller E, Huerta P, Segarra F, Matino E, Enrique A, Adema JM. Undiagnosed (corrected) cases of obstructive sleep apnoea syndrome: a new reason for involvement of otorhinolaryngologists. Acta Otorrinolaringol Esp. 2008;59:62–9.
Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33.
Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2(Suppl 1):S4–11.
Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983;111:47–54.
Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–7.
Steensma DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842–8.
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–40.
Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–803.
Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–61.
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–7.
Maurillo L, Venditti A, Spagnoli A, Gaidano G, Ferrero D, Oliva E, et al. Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian compassionate program. Cancer. 2012;118:1014–22.
O’Connor OA, Masszi T, Savage KJ, et al. Belinostat, a novel pan-histone deacetylase inhibitor (HDACi), in relappsed or or refractory perpheral T cell lymphoma (R/R PTCL: results from the BELIEF trial). J Clin Oncol. 2013;31(15s):8507.
Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109:31–9.
Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–15.
Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–34.
Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–7.
San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–206.
Stathis A, Bertoni F. BET proteins as targets for anticancer treatment. Cancer Discov. 2018;8:24–36.
Fukazawa H, Masumi A. The conserved 12-amino acid stretch in the inter-bromodomain region of BET family proteins functions as a nuclear localization signal. Biol Pharm Bull. 2012;35:2064–8.
Devaiah BN, Lewis BA, Cherman N, Hewitt MC, Albrecht BK, Robey PG, et al. BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc Natl Acad Sci U S A. 2012;109:6927–32.
Devaiah BN, Singer DS. Two faces of brd4: mitotic bookmark and transcriptional lynchpin. Transcription. 2013;4:13–7.
Mochizuki K, Nishiyama A, Jang MK, Dey A, Ghosh A, Tamura T, et al. The bromodomain protein Brd4 stimulates G1 gene transcription and promotes progression to S phase. J Biol Chem. 2008;283:9040–8.
You J, Li Q, Wu C, Kim J, Ottinger M, Howley PM. Regulation of aurora B expression by the bromodomain protein Brd4. Mol Cell Biol. 2009;29:5094–103.
Jiang YW, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway JW, Conaway RC, et al. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc Natl Acad Sci U S A. 1998;95:8538–43.
Denis GV, McComb ME, Faller DV, Sinha A, Romesser PB, Costello CE. Identification of transcription complexes that contain the double bromodomain protein Brd2 and chromatin remodeling machines. J Proteome Res. 2006;5:502–11.
Denis GV, Vaziri C, Guo N, Faller DV. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ. 2000;11:417–24.
LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30:51–60.
Loven J, Hoke HA, Lin CY, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–34.
Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–8.
Coude MM, Braun T, Berrou J, et al. BET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cells. Oncotarget. 2015;6:17698–712.
Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82.
Segura MF, Fontanals-Cirera B, Gaziel-Sovran A, Guijarro MV, Hanniford D, Zhang G, et al. BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy. Cancer Res. 2013;73:6264–76.
Zhang Z, Ma P, Jing Y, Yan Y, Cai MC, Zhang M, et al. BET Bromodomain inhibition as a therapeutic strategy in ovarian cancer by downregulating FoxM1. Theranostics. 2016;6:219–30.
Zhu H, Bengsch F, Svoronos N, Rutkowski MR, Bitler BG, Allegrezza MJ, et al. BET bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16:2829–37.
French CA. NUT midline carcinoma. Cancer Genet Cytogenet. 2010;203:16–20.
• Stathis A, Zucca E, Bekradda M, et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500 This article represents the first clinical proof-of-concept that targeting BRD4-NUT with a BET inhibitor results in antitumor activity in NMC.
• Lewin J, Soria JC, Stathis A, et al. hase I trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins in patients with selected advanced solid tumors. J Clin Oncol. 2018 JCO2018782292. This is the first phase I study in patients with solid tumors with a BET inhibitor to be reported in complete form. The study also reported clinical activity of BET inhibition in NMC with 3 over 10 patients treated achieving a response.
O’Dwyer PJ, Piha-Paul SA, French C et al. GSK525762 a selective bromodomain (BRD) and extraterminal protein (BET) inhibitor: results from a part 1 of a phase I/II open label single agent study in patients with NUT midline carcinoma (NMC) and other cancers. Cancer Res. 2016;76(14 Supplement):CT014–CT014.
Shapiro GI, Dowlati A, LoRusso PM, et al. Clinical efficacy of the BET bromodomain inhibitor TEN-010 in an open-label substudy with patients with documented NUT midline carcinoma (NMC). In: AACR-NCI-EORTC international conference: molecular targets and cancer therapeutics: November 5–9; 2015.
Aftimos PJ, Bechter O, Awada A, et al. Phase I first-in-men trial of a novel bromodomain and extraterminal domain (BET) inhibitor (BI 894999) in patients (pts) with advanced malignancies. J Clin Oncol. 2017;35:15.
Falchook G, Talpaz M, Mita M, et al. Abstract A093: Phase I/II study of INCB054329, a bromodomain and extraterminal (BET) protein inhibitor, in patients (pts) with advanced malignancies. Mol Cancer Ther. 1 2018;(17) (1 Supplement) A093.
Dombret H, Preudhomme C, Berthon C, et al. A phase I study of the BET-bromodomain inhibitor OTX015 in patients with advanced acute leukemia. Blood. 2014;14:117.
• Berthon C, Raffoux E, Thomas X, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 2016;3:e186–95 This is the first report of activity of BET inhibition in patients with acute leukemia.
Dawson M, Stein EM, Huntly BJP, et al. A phase I study of GSK525762, a selective bromodomain (BRD) and extraterminal protein (BET) inhibitor: results from part 1 of phase I/II open label single agent study in patients with acute myeloid leukemia (AML). Blood. 2017;130:1377.
Borthakur G, Wolff JE, Aldoss I, et al. First-in-human study of ABBV-075 (mivibresib), a pan-inhibitor of bromodomain and extraterminal (BET) proteins in patients (pts) with relappsed/refractory (RR) acute myeloid leukemia (AML): preliminary data. J Clin Oncol. 2018;36, n(15 Suppl):7019.
Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3:e196–204.
Blum KA, Abramson J, Maris M, et al. A phase I study of CPI-0610, a bromodomain and extraterminal protein (BET) inhibitor in patients with relapsed or refractory lymphoma. Ann Onco. 2018;29(3 Suppl):mdy048.
Ramadoss M, Mahadevan V. Targeting the cancer epigenome: synergistic therapy with bromodomain inhibitors. Drug Discov Today. 2018;23(1):76–89.
Kharenko OA, Hansen HC. Novel approaches to targeting BRD4. Drug Discov Today Technol. 2017;24:19–24.
Quian C, Zhou MM. SET domain protein lysine methyltransferases: structure, specificity and catalysis. Cell Mol Life Svi. 2006;63(23):2755–3.
Velichutina I, Shaknovich R, Geng H, Johnson NA, Gascoyne RD, Melnick AM, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood. 2010;116:5247–55.
Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9.
Stazi G, Zwergel C, Mai A, Valente S. EZH2 inhibitors: a patent review (2014–2016). Expert Opin Ther Pat. 2017;27:797–813.
Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4:124–31.
Gulati N, Beguelin W, Giulino-Roth L. Enhancer of zeste homolog 2 (EZH2) inhibitors. Leuk Lymphoma. 2018;59:1574–85.
Khan SN, Jankowska AM, Mahfouz R, Dunbar AJ, Sugimoto Y, Hosono N, et al. Multiple mechanisms deregulate EZH2 and histone H3 lysine 27 epigenetic changes in myeloid malignancies. Leukemia. 2013;27:1301–9.
Jankowska AM, Makishima H, Tiu RV, Szpurka H, Huang Y, Traina F, et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118:3932–41.
Wang X, Dai H, Wang Q, Wang Q, Xu Y, Wang Y, et al. EZH2 mutations are related to low blast percentage in bone marrow and −7/del(7q) in de novo acute myeloid leukemia. PLoS One. 2013;8:e61341.
Ntziachristos P, Tsirigos A, Van Vlierberghe P, et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med. 2012;18:298–301.
Koh CM, Iwata T, Zheng Q, Bethel C, Yegnasubramanian S, De Marzo AM. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget. 2011;2:669–83.
Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–9.
Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–35.
Fujii S, Tokita K, Wada N, Ito K, Yamauchi C, Ito Y, et al. MEK-ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011;30:4118–28.
Coe BP, Thu KL, Aviel-Ronen S, Vucic EA, Gazdar AF, Lam S, et al. Genomic deregulation of the E2F/Rb pathway leads to activation of the oncogene EZH2 in small cell lung cancer. PLoS One. 2013;8:e71670.
Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-cell lymphomas. Cancer Cell. 2012;22:506–23.
Yan J, Ng SB, Tay JL, et al. EZH2 overexpression in natural killer/T-cell lymphoma confers growth advantage independently of histone methyltransferase activity. Blood. 2013;121:4512–20.
Lue JK, Amengual JE. Emerging EZH2 inhibitors and their application in lymphoma. Curr Hematol Malig Rep. 2018;13:369–82.
Miranda TB, Cortez CC, Yoo CB, Liang G, Abe M, Kelly TK, et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther. 2009;8:1579–88.
Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci U S A. 2012;109:21360–5.
McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12.
Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ, Klaus CR, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8:890–6.
Knutson SK, Warholic NM, Wigle TJ, Klaus CR, Allain CJ, Raimondi A, et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A. 2013;110:7922–7.
Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho YJ, et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18:316–28.
• Italiano A, Soria JC, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19:649–59 This study reports in complete form results of a phase I study with the EZH2 inhibitor tazemetostat in patients with non-Hodkins lymphoma and solid tumors.
• Morschhauser F, Salles G, McKay P, et al. Interim report from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor: clinical activity and favorable safety in patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Hematol Oncol. 2017;35(S2):24–25. This is a preliminary report of a phase II study of tazemetostat in patients with follicular and DLBCL. Results of this study are important in that they clearly show a very high clinical activity of the compound in patients with relapsed or refractory disease (in particular patients with mutated EZH2 follicular lymphoma).
Schoffski P, Agulnik M, Stacchiotti S, et al. Phase II multicenter study of the EZH2 inhibitor tazemetostat in adults with synovial sarcoma (NCT02601950). J Clin Oncol. 2017;35, n(15 Suppl):11057.
Maruyama D, Tobinai K, Makita S, et al. Firts-in-human study of the EZH1/2 dual inhibitor DS-3201b in patients with relapsed or refractory non-Hodgkin lymphomas-preliminary results. Blood. 2017;130(suppl 1):4070.
Harb W, Abramson J, Lunning M, et al. A hase I study of CPI-1205 a small molecule inhibitor of EZH2, preliminary safety in patients with B-cell lymphomas. Ann Oncol. 2018;29(3 Suppl):mdy048.001.
Yap TA, Winter JN, Leonard JP, et al. A phase I study of GSK2816126, an enhancer of zeste homolog 2 (EZH2) inhibitor, in patients (pts) with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), other non-Hodgkin lymphomas (NHL), transformed follicular lymphoma (tFL), solid tumors and multiple myeloma (MM). Blood. 2016;128:4203.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sofia Genta declares that she has no conflict of interest.
Maria Cristina Pirosa declares that she has no conflict of interest.
Anastasios Stathis has received research funding (paid to his institution) from Amgen, Bayer, Novartis, Pfizer, Roche, and MEI Pharma, and has received travel grants from AbbVie and PharmaMar.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evolving Therapies
Rights and permissions
About this article
Cite this article
Genta, S., Pirosa, M.C. & Stathis, A. BET and EZH2 Inhibitors: Novel Approaches for Targeting Cancer. Curr Oncol Rep 21, 13 (2019). https://doi.org/10.1007/s11912-019-0762-x
Published:
DOI: https://doi.org/10.1007/s11912-019-0762-x