Abstract
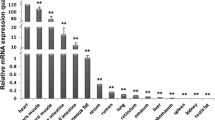
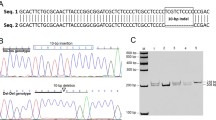
GPIHBP1 is expressed restrictively in capillary endothelial cells, but the transcriptional regulation mechanisms of GPIHBP1 in capillaries are still unknown. Here, we analyzed the promoter activities of porcine GPIHBP1 using dual luciferase reporter assays and detected single nucleotide polymorphisms (SNPs) in the promoter region. Activity analysis discovered that the sequence from–187 to +174 is a basal promoter region, the sequence from–187 to–582 is a positive regulation region, whereas the sequence from–582 to–1321 is a negative regulation region. 11 SNPs were found in the promoter region of porcine GPIHBP1, and the g.-796G>A SNP within the NF-l-binding site is associated with pig back fat thickness. The work performed here will serve as a valuable resource for discovering the expression regulation mechanism of GPIHBP1 and promoting further development of pig as a model organism for human cardiovascular disease research.
Similar content being viewed by others
References
Havel, R.J. and Kane, J.P., Introduction: structure and metabolism of plasma lipoproteins, in The Metabolic and Molecular Bases of Inherited Disease, Scriver, C.R., Ed., New York: McGraw-Hill, 2001, pp. 2705–2716.
Olivecrona, T. and Olivecrona, G., The ins and outs of adipose tissue, in Cellular Lipid Metabolism, Ehnholm, C., Ed., Heidelberg: Springer-Verlag, 2009, pp. 315–369.
Nikolaev, I.V., Mulyukova, R.V., Kayumova, L.R., et al., Analysis of interactions of lipid metabolism alleles in dyslipidemia, Russ. J. Genet.: Appl. Res., 2014, vol. 5, no. 4, pp. 313–321.
Obunike, J.C., Lutz, E.P., Li, Z., Paka, L., Katopodis, T., et al., Transcytosis of lipoprotein lipase across cultured endothelial cells requires both heparan sulfate proteoglycans and the very low density lipoprotein receptor, J. Biol. Chem., 2001, vol. 276, no. 12, pp. 8934–8941.
Ioka, R.X., Kang, M.-J., Kamiyama, S., Kim, D.-H., Magoori, K., et al., Expression cloning and characterization of a novel glycosylphosphatidylinositolanchored high density lipoprotein-binding protein, GPI-HBP1, J. Biol. Chem., 2003, vol. 278, no. 9, pp. 7344–7349.
Beigneux, A.P., Davies, B.S., Gin, P., et al., Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons, Cell Metab., 2007, vol. 5, no. 4, pp. 279–291.
Davies, B.S., Beigneux, A.P., Barnes, R.H., et al., GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries, Cell Metab., 2010, vol. 12, no. 1, pp. 42–52.
Gin, P., Beigneux, A.P., Davies, B., et al., Normal binding of lipoprotein lipase, chylomicrons, and apo-AV to GPIHBP1 containing a G56R amino acid substitution, BBA-Mol. Cell Biol. L., 2007, vol. 1771, no. 12, pp. 1464–1468.
Gerhard, D.S., Wagner, L., Feingold, E.A., et al., The status, quality, and expansion of the NIH full-length cDNA project, Genome Res., 2004, vol. 14, no. 14, pp. 2121–2127.
Gibbs, R.A., Weinstock, G.M., Metzker, M.L., et al., Genome sequence of the Brown Norway rat yields insights into mammalian evolution, Nature, 2004, vol. 428, no. 6982, pp. 493–521.
Thierry-Mieg, D. and Thierry-Mieg, J., AceView: a comprehensive cDNA-supported gene and transcripts, Genome Biol., 2006, vol. 7, p. S12.
Xu, H.M., Tao, X.L., Wei, Y.Y., et al., Cloning of porcine GPIHBP1 gene and its tissue expression pattern and genetic effect on adipose traits, Gene, 2015, vol. 557, no. 2, pp. 146–153.
Wang, J. and Hegele, R.A., Homozygous missense mutation (G56R) in glycosylphosphatidylinositolanchored high-density lipoprotein-binding protein 1 (GPI-HBP1) in two siblings with fasting chylomicronemia (MIM 144650), Lipids Health Dis., 2007, vol. 6, no. 1, pp. 1200–1205.
Beigneux, A.P., Franssen, R., Bensadoun, A., et al., Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase, Arterioscler. Thromb. Vasc. Biol., 2009, vol. 29, no. 6, pp. 956–962.
Franssen, R., Young, S.G., Peelman, F., et al., Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects, Circ. Cardiovasc. Genet., 2010, vol. 3, no. 2, pp. 169–178.
Olivecrona, G., Ehrenborg, E., Semb, H., et al., Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia, J. Lipid Res., 2010, vol. 51, no. 6, pp. 1535–1545.
Charrière, S., Peretti, N., Bernard, S., et al., GPIHBP1 C89F neomutation and hydrophobic C-terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia, J. Clin. Endocrinol. Metab., 2011, vol. 96, no. 96, pp. e1675–e1679.
Coca-Prieto, I., Kroupa, O., Gonzalez-Santos, P., et al., Childhood-onset chylomicronaemia with reduced plasma lipoprotein lipase activity and mass: identification of a novel GPIHBP1 mutation, J. Int. Med., 2011, vol. 270, no. 3, pp. 224–228.
Olafsen, T., Young, S.G., Davies, B.S., et al., Unexpected expression pattern for glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) in mouse tissues revealed by positron emission tomography scanning, J. Biol. Chem., 2010, vol. 285, no. 285, pp. 39239–39248.
Davies, B.S., Waki, H., Beigneux, A.P., et al., The expression of GPIHBP1, an endothelial cell binding site for lipoprotein lipase and chylomicrons, is induced by peroxisome proliferator-activated receptor-γ, Mol. Endocrinol., 2008, vol. 22, no. 11, pp. 2496–2504.
Lunney, J.K., Advances in swine biomedical model genomics, Int. J. Biol. Sci., 2007, vol. 3, no. 3, pp. 179–184.
Simon, G.A. and Maibach, H.I., The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations–an overview, Skin Pharmacol. Physiol., 2000, vol. 13, no. 5, pp. 229–234.
Jiang, Y.Z., Cen, W.M., Xing, S.H., et al., Tissue expression pattern and polymorphism of G0S2 gene in porcine, Gene, 2014, vol. 539, no. 1, pp. 173–179.
Kuscu, C., Evensen, N., Kim, D., et al., Transcriptional and epigenetic regulation of KIAA1199 gene expression in human breast cancer, PLoS One, 2012, vol. 7, no. 9, p. e44661.
Zheng, J., Long, L., Hu, J., et al., Isolation and sequence analysis of Sox genes in the red crucian carp (Carassius carassius red variety), Russ. J. Genet., 2008, vol. 44, no. 11, pp. 1325–1330.
Bell, D.M., Leung, K.K., Wheatley, S.C., et al., SOX9 directly regulates the type-II collagen gene, Nat. Genet., 1997, vol. 16, no. 2, pp. 174–178.
Zhu, L. and Li, X.-W., PCR-RFLP polymorphisms and genetic effects of MyoD gene in different pig breeds, Acta Vet. Zootech. Sin., 2005, vol. 36, no. 8, pp. 761–766.
Bagwell, A.M., Bailly, A., Mychaleckyj, J.C., et al., Comparative genomic analysis of the HNF-4α transcription factor gene, Mol. Genet. Metab., 2004, vol. 81, no. 2, pp. 112–121.
Horikawa, Y., Oda, N., Cox, N.J., et al., Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus, Nat. Genet., 2000, vol. 26, no. 26, pp. 163–175.
Silander, K., Mohlke, K.L., Scott, L.J., et al., Genetic variation near the hepatocyte nuclear factor-4α gene predicts susceptibility to type 2 diabetes, Diabetes, 2004, vol. 53, no. 4, pp. 1141–1149.
Bagwell, A.M., Bento, J.L., Mychaleckyj, J.C., et al., Genetic analysis of HNF4A polymorphisms in Caucasian-American type 2 diabetes, Diabetes, 2005, vol. 54, no. 4, pp. 1185–1190.
Hani, E., Suaud, L., Boutin, P., et al., A missense mutation in hepatocyte nuclear factor-4 alpha, resulting in a reduced transactivation activity, in human lateonset non-insulin-dependent diabetes mellitus, J. Clin. Invest., 1998, vol. 101, no. 3, pp. 521–526.
Zhu, Q., Yamagata, K., Miura, A., et al., T130I mutation in HNF-4α gene is a loss-of-function mutation in hepatocytes and is associated with late-onset type 2 diabetes mellitus in Japanese subjects, Diabetologia, 2003, vol. 46, no. 4, pp. 567–573.
Odom, D.T., Zizlsperger, N., Gordon, D.B., et al., Control of pancreas and liver gene expression by HNF transcription factors, Science, 2004, vol. 303, no. 5662, pp. 1378–1381.
Demina, Y.P., Miroshnikova, V.V., Mayorov, N.V., et al., Reduction of the level of LXRβ mRNA and PPARγ mRNA in macrophages stimulated with a macrophage colony-stimulating factor in patients with atherosclerosis, Russ. J. Genet.: Appl. Res., 2015, vol. 5, no. 2, pp. 155–158.
Graves, R., Tontonoz, P., Ross, S., and Spiegelman, B., Identification of a potent adipocyte-specific enhancer: involvement of an NF-1-like factor, Genes Dev., 1991, vol. 5, no. 3, pp. 428–437.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Chen, J., Chen, Y., Wei, Y. et al. Activities Analysis and Polymorphisms Identification of GPIHBP1 Promoter Region in Porcine. Russ J Genet 54, 680–686 (2018). https://doi.org/10.1134/S1022795418060042
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795418060042