Abstract
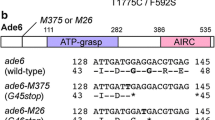
Nonlethal nonsense mutations obtained earlier in the essential gene SUP45 encoding the translation termination factor eRF1 in the yeast Saccharomyces cerevisiae were further characterized. Strains carrying these mutations retain the viability, since the full-length eRF1 protein is present in these strains, although in decreased amounts as compared to wild-type cells, together with a trucated eRF1. All nonsense mutations are likely to be located in a weak termination context, because a change in the stop codon UGAA (in the case of mutation sup45-107) to UAGA (sup45-107.2) led to the alteration of the local context from a weak to strong and to the lethality of the strain carrying sup45-107.2. All nonsense mutations studied are characterized by thermosensitivity expressed as cell mortality after cultivation at 37°C. When grown under nonpermissive conditions (37°C), cells of nonsense mutants sup45-104, sup45-105, and sup45-107 display a decrease in the amount of the truncated eRF1 protein without reduction in the amount of the full-length eRF1 protein. The results of this study suggest that the N-terminal eRF1 fragment is indispensable for cell viability of nonsense mutants due to the involvement in termination of translation.
Similar content being viewed by others
References
Kisselev, L., Ehrenberg, M., and Frolova, L., Termination of Translation: Interplay of mRNA, rRNAs and Release Factors?, EMBO J., 2003, vol. 22, pp. 175–182.
Inge-Vechtomov, S., Zhouravleva, G., and Philippe, M., Eukaryotic Release Factors (eRFs) History, Biol. Cell, 2003, vol. 95, pp. 195–209.
Frolova, L., Le Goff, X., Rasmussen, H.H., et al., A Highly Conserved Eukaryotic Protein Family Possessing Properties of Polypeptide Chain Release Factor, Nature, 1994, vol. 372, pp. 701–703.
Zhouravleva, G., Frolova, L., Le Goff, X., et al., Termination of Translation in Eukaryotes Is Governed by Two Interacting Polypeptide Chain Release Factors, eRF1 and eRF3, EMBO J., 1995, vol. 14, pp. 4065–4072.
Stansfield, I., Jones, K.M., Kushnirov, V.V., et al., The Products of the SUP45 (eRF1) and SUP35 Genes Interact to Mediate Translation Termination in S. cerevisiae, EMBO J., 1995, vol. 14, pp. 4365–4373.
Eurwilaichitr, L., Graves, F.M., Stansfield, I., and Tuite, M.F., The C-Terminus of eRF1 Defines a Functionally Important Domain for Translation Termination in S. cerevisiae, Mol. Microbiol., 1999, vol. 32, pp. 485–496.
Merkulova, T.I., Frolova, L.Y., Lazar, M., et al., C-Terminal Domains of Human Translation Termination Factors eRF1 and eRF3 Mediate Their in Vivo Interaction, FEBS Lett., 1999, vol. 443, pp. 41–47.
Salas-Marco, J. and Bedwell, D.M., GTP Hydrolysis by eRF3 Facilitates Stop Codon Decoding during Eukaryotic Translation Termination, Mol. Cell. Biol., 2004, vol. 24, pp. 7769–7778.
Ito, K., Frolova, L., Seit-Nebi, A., et al., Omnipotent Decoding Potential Resides in Eukaryotic Translation Termination Factor eRF1 of Variant-Code Organisms and Is Modulated by the Interactions of Amino Acid Sequences within Domain 1, Proc. Natl. Acad. Sci. USA, 2002, vol. 99, pp. 8494–8499.
Salas-Marco, J., Fan-Minogue, H., Kallmeyer, A.K., et al., Distinct Paths to Stop Codon Reassignment by the Variant-Code Organisms Tetrahymena and Euplotes, Mol. Cell Biol., 2006, vol. 26, pp. 438–447.
Inge-Vechtomov, S.G. and Andrianova, V.M., Recessive Super-Suppressors in Yeast, Genetika (Moscow), 1970, vol. 6, no. 1, pp. 103–116.
Culbertson, M.R., Gaber, R.F., and Cummins, C.M., Frameshift Suppression in S. cerevisiae: V. Isolation and Genetic Properties of Nongroup-Specific Suppressors, Genetics, 1982, vol. 102, pp. 361–378.
Kulikov, V.N., Tikhodeev, O.N., Forafonov, F.S., et al., Partial Inactivation of Translation Termination Factors Causes Suppression of Frameshift Mutations in the Yeast Saccharomyces cerevisiae, Russ. J. Genet., 2001, vol. 37, no. 5, pp. 486–493.
Mathison, L. and Culbertson, M.R., Suppressible and Nonsuppressible +1 G-C Base Pair Insertions Induced by ICR-170 at the his4 Locus in S. cerevisiae, Mol. Cell. Biol., 1985, vol. 5, pp. 2247–2256.
Kikuchi, Y., Shimatake, H., and Kikuchi, A., A Yeast Gene Required for the G1-to-S Transition Encodes a Protein Containing an A-Kinase Target Site and GTPase Domain, EMBO J., 1988, vol. 7, pp. 1175–1182.
Borkhsenius, A.S. and Inge-Vechtomov, S.G., The Role of SUR35 and SUR45 Genes in Controlling Saccharomycetes Cell Cycle, Dokl. Akad. Nauk, 1997, vol. 353, pp. 553–556.
Tikhomirova, V.L. and Inge-Vechtomov, S.G., Sensitivity of sup35 and sup45 Suppressor Mutants in S. cerevisiae to the Anti-Microtubule Drug Benomyl, Curr. Genet., 1996, vol. 30, pp. 44–49.
Borchsenius, A.S., Tchourikova, A.A., and Inge-Vechtomov, S.G., Recessive Mutations in SUP35 and SUP45 Genes Coding for Translation Release Factors Affect Chromosome Stability in S. cerevisiae, Curr. Genet., 2000, vol. 37, pp. 285–291.
Borkhsenius, A.S., Repnevskaya, M.V., Kurishko, K., and Inge-Vechtomov, S.G., Assosiation between Defects of Karyogamy and Translation Termination in Yeast Saccharomyces cerevisiae, Russ. J. Genet., 2005, vol. 41, no. 2, pp. 122–129.
Bailleul, P.A., Newnam, G.P., Steenbergen, J.N., and Chernoff, Y.O., Genetic Study of Interactions between the Cytoskeletal Assembly Protein Sla1 and Prion-Forming Domain of the Release Factor Sup35 (eRF3) in S. cerevisiae, Genetics, 1995, vol. 153, pp. 81–94.
Valouev, I.A., Kushnirov, V.V., and Ter Avanesyan, M.D., Yeast Polypeptide Chain Release Factors eRF1 and eRF3 are Involved in Cytoskeleton Organization and Cell Cycle Regulation, Cell Motil. Cytoskeleton, 2002, vol. 52, pp. 161–173.
Inge-Vechtomov, S.G. and Andrianova, V.M., New Type of Supersupressors in Yeasts, in Molekulyarnye mekhanizmy geneticheskikh protsesssov (Molecular Mechanisms of Genetic Processes), Moscow: Nauka, 1972, pp. 189–195.
Ter-Avanesyan, M.D. and Inge-Vechtomov, S.G., Interaction of Dominant and Recessive Suppressors in Yeast Saccharomyces cerevisiae, Genetika (Moscow), 1980, vol. 16, no. 1, pp. 86–94.
Surguchov, A.P., Fominykch, E.S., Smirnov, V.N., et al., Further Characterization of Recessive Suppression in Yeast: Isolation of the Low-Temperature Sensitive Mutant of S. cerevisiae Defective in the Assembly of 60 S Ribosomal Subunit, Biochim. Biophys. Acta, 1981, vol. 654, pp. 149–155.
Chabelskaya, S., Kiktev, D., Inge-Vechtomov, S., et al., Nonsense Mutations in the Essential Gene SUP35 of S. cerevisiae Are Non-Lethal, Mol. Genet. Genomics, 2004, vol. 272, pp. 297–307.
Moskalenko, S.E., Chabelskaya, S.V., Inge-Vechtomov, S.G., et al., Viable Nonsense Mutants for the Essential Gene SUP45 of S. cerevisiae, BMC Mol. Biol., 2003, vol. 4, p. 2.
Sikorski, R.S. and Hieter, P., A System of Shuttle Vectors and Yeast Host Strains Designed for Efficient Manipulation of DNA in S. cerevisiae, Genetics, 1989, vol. 122, pp. 19–27.
Sherman, F., Fink, G.R., and Hicks, J.B., Laboratory Course Manual for Methods in Yeast Genetics, New York: Cold Spring Harbor Lab., 1986.
Gietz, R.D., Schiestl, R.H., Willems, A.R., and Woods, R.A., Studies on the Transformation of Intact Yeast Cells by the LiAc/SS-DNA/PEG Procedure, Yeast, 1995, vol. 11, pp. 355–360.
Inoue, H., Nojima, H., and Okayama, H., High Efficiency Transformation of E. coli with Plasmids, Gene, 1990, vol. 96, pp. 23–28.
Kaiser, C., Michaelis, S., and Mitchell, A., Methods in Yeast Genetics, New York: Cold Spring Harbor Lab., 1994.
Didichenko, S.A., Ter Avanesyan, M.D., and Smirnov, V.N., Ribosome-Bound EF-1 Alpha-Like Protein of Yeast S. cerevisiae, Eur. J. Biochem., 1991, vol. 198, pp. 705–711.
Laemmli, U.K., Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4, Nature, 1970, vol. 227, pp. 680–685.
Towbin, H., Staehelin, T., and Gordon, J., Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications, Proc. Natl. Acad. Sci. USA, 1979, vol. 76, pp. 4350–4354.
Tate, W.P. and Mannering, S.A., Three, Four or More: The Translational Stop Signal at Length, Mol. Microbiol., 1996, vol. 21, pp. 213–219.
Kisselev, L.L. and Buckingham, R.H., Translational Termination Comes of Age, Trends Biochem. Sci., 2000, vol. 25, pp. 561–566.
Bertram, G., Innes, S., Minella, O., et al., Endless Possibilities: Translation Termination and Stop Codon Recognition, Microbiology, 2001, vol. 147, pp. 255–269.
Poole, E.S., Brown, C.M., and Tate, W.P., The Identity of the Base Following the Stop Codon Determines the Efficiency of in Vivo Translational Termination in E. coli, EMBO J., 1995, vol. 14, pp. 151–158.
Tate, W.P., Poole, E.S., and Mannering, S.A., Hidden Infidelities of the Translational Stop Signal, Prog. Nucleic Acid Res. Mol. Biol., 1996, vol. 52, pp. 293–335.
Brown, C.M., Stockwell, P.A., Trotman, C.N., and Tate, W.P., The Signal for the Termination of Protein Synthesis in Prokaryotes, Nucleic Acids Res., 1990, vol. 18, pp. 2079–2086.
McCaughan, K.K., Brown, C.M., Dalphin, M.E., et al., Translational Termination Efficiency in Mammals Is Influenced by the Base Following the Stop Codon, Proc. Natl. Acad. Sci. USA, 1995, vol. 92, pp. 5431–5435.
Ozawa, Y., Hanaoka, S., Saito, R., et al., Comprehensive Sequence Analysis of Trnaslation Termination Sites in Various Eukaryotes, Gene, 2002, vol. 300, pp. 79–87.
Poole, E.S., Brimacombe, R., and Tate, W.P., Decoding the Translational Termination Signal: The Polypeptide Chain Release Factor in E. coli Crosslinks to the Base Following the Stop Codon, RNA, 1997, vol. 3, pp. 974–982.
Poole, E.S., Major, L.L., Mannering, S.A., and Tate, W.P., Translational Termination in Escherichia coli: Three Bases Following the Stop Codon Crosslink to Release Factor 2 and Affect the Decoding Efficiency of UGA-Containing Signals, Nucleic Acids Res., 1998, vol. 26, pp. 954–960.
Mottagui-Tabar, S., Tuite, M.F., and Isaksson, L.A., The Influence of 5′ Codon Context on Translation Termination in S. cerevisiae, Eur. J. Biochem., 1998, vol. 257, pp. 249–254.
Cassan, M. and Rousset, J.P., UAG Readthrough in Mammalian Cells: Effect of Upstream and Downstream Stop Codon Contexts Reveal Different Signals, BMC Mol. Biol., 2001, vol. 2, p. 3.
Namy, O., Hatin, I., and Rousset, J.P., Impact of the Six Nucleotides Downstream of the Stop Codon on Translation Termination, EMBO Rep., 2001, vol. 2, pp. 787–793.
Namy, O., Duchateau-Nguyen, G., and Rousset, J.P., Translational Readthrough of the PDE2 Stop Codon Modulates cAMP Levels in S. cerevisiae, Mol. Microbiol., 2002, vol. 43, pp. 641–652.
Liu, Q., Comparative Analysis of Base Biases around the Stop Codons in Six Eukaryotes, Biosystems, 2005, vol. 81, pp. 281–289.
Tork, S., Hatin, I., Rousset, J.P., and Fabret, C., The Major 5′ Determinant in Stop Codon Read-Through Involves Two Adjacent Adenines, Nucleic Acids Res., 2004, vol. 32, pp. 415–421.
Zhouravleva, G.A., Moskalenko, S.E., Shabel’skaya, S.V., et al., Increased tRNA Concentration in Yeast Containing Mutant Termination Translation Factors eRF1 and eRF3, Mol. Biol. (Moscow), 2006, vol. 40, pp. 724–730.
Bonetti, B., Fu, L., Moon, J., and Bedwell, D.M., The Efficiency of Translation Termination Is Determined by a Synergistic Interplay between Upstream and Downstream Sequences in S. cerevisiae, J. Mol. Biol., 1995, vol. 251, pp. 334–345.
Stansfield, I., Eurwilaichitr, L., Akhmaloka, and Tuite, M.F., Depletion in the Levels of the Release Factor eRF1 Causes a Reduction in the Efficiency of Translation Termination in Yeast, Mol. Microbiol., 2006, vol. 20, pp. 1135–1143.
Breining, P. and Piepersberg, W., Yeast Omnipotent Suppressor SUP1 (SUP45): Nucleotide Sequence of the Wild Type and a Mutant Gene, Nucleic Acids Res., 1986, vol. 14, pp. 5187–5197.
Bertram, G., Bell, H.A., Ritchie, D.W., et al., Terminating Eukaryote Translation: Domain 1 of Release Factor eRF1 Functions in Stop Codon Recognition, RNA, 2000, vol. 6, pp. 1236–1247.
Kawakami, K. and Nakamura, Y., Autogenous Suppression of an Opal Mutation in the Gene Encoding Peptide Chain Release Factor 2, Proc. Natl. Acad. Sci. USA, 1990, vol. 87, pp. 8432–8436.
Stansfield, I., Akhmaloka, and Tuite, M.F., A Mutant Allele of the SUP45 (SAL4) Gene of S. cerevisiae Shows Temperature-Dependent Allosuppressor and Omnipotent Suppressor Phenotypes, Curr. Genet., 1995, vol. 27, pp. 417–426.
Cosson, B., Couturier, A., Chabelskaya, S., et al., Poly(A)-Binding Protein Acts in Translation Termination via Eukaryotic Release Factor 3 Interaction and Does not Influence [PSI+] Propagation, Mol. Cell. Biol., 2002, vol. 22, pp. 3301–3315.
Stansfield, I., Kushnirov, V.V., Jones, K.M., and Tuite, M.F., A Conditional-Lethal Translation Termination Defect in a sup45 Mutant of the Yeast S. cerevisiae, Eur. J. Biochem., 1997, vol. 245, pp. 557–563.
Moskalenko, S.E., Zhouravleva, G.A., Soom, M.Ya., et al, Characterization of Missense Mutations in the SUR45 Gene of Saccharomyces cerevisiae Encoding Translation Termination Factor eRF1, Genetika (Moscow), 2004, vol. 40, no. 5, pp. 468–484.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.A. Zhouravleva, S.E. Moskalenko, O.A. Murina, S.G. Inge-Vechtomov, 2007, published in Genetika, 2007, Vol. 43, No. 10, pp. 1363–1371.
Rights and permissions
About this article
Cite this article
Zhouravleva, G.A., Moskalenko, S.E., Murina, O.A. et al. Viable nonsense mutants for the SUP45 gene in the yeast Saccharomyces cerevisiae are lethal at increased temperature. Russ J Genet 43, 1139–1146 (2007). https://doi.org/10.1134/S1022795407100079
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1022795407100079