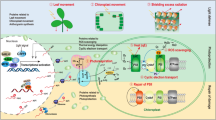
Abstract
In plant cells the homeostatic control of energy balance involves the production and recycling of adenylates with macroergic bonds, ATP and ADP. The maintenance of anabolic processes requires the relative saturation of the adenylate pool with high energy phosphoanhydride bonds. The bulk of ATP synthesis is carried out both in mitochondria and in chloroplasts while optimal ATP levels within other cell compartments are maintained by adenylate kinases (AK). AK activity was recently found in cytosol, mitochondria, plastids and the nucleus. ATP synthesis in energy-producing organelles, as well as redistribution of nutrients among cellular compartments, requires fine-tuned regulation of ion homeostasis. A special role in energy metabolism is played by autophagy, a process of active degradation of unwanted and/or damaged cell components and macromolecules within the central lytic vacuole. So-called constitutive autophagy controls the quality of cellular contents under favorable conditions, i.e., when the cellular energy status is high. Energy depletion can lead to the activation of the pro-survival process of autophagic removal and utilization of damaged structures; the breakdown products are then used for ATP regeneration and de novo synthesis of macromolecules. Mitophagy and chlorophagy maintain the populations of healthy and functional energy-producing “stations”, preventing accumulation of defective mitochondria and chloroplasts as potential sources of dangerous reactive oxygen species. However, the increase of autophagic flux above a threshold level can lead to the execution of the vacuolar type of programmed cell death (PCD). In this case autophagy also contributes to preservation of energy through support of the outflow of nutrients from dying cells to healthy neighboring tissues. In plants, two central protein kinases, SnRK1 (Snf1-related protein kinase 1) and TOR (target of rapamycin), are responsible for the regulation of the metabolic switch between anabolic and catabolic pathways. TOR promotes the energy-demanding metabolic reactions in response to nutrient availability and simultaneously suppresses catabolism including autophagy. SnRK1, the antagonist of TOR, senses a decline in cellular energy supply and reacts by inducing autophagy through several independent pathways. Here, we provide an overview of the recent knowledge about the interplay between SnRK1 and TOR, autophagy and PCD in course of the regulation of energy balance in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Plant growth and development are energy demanding processes. Depending on environmental conditions, or upon shifts of morphogenetic programs related to acceleration of seedling growth or to senescence, a local short-term or a deep long-term energy deprivation can emerge. Autophagy (from the Ancient Greek for “self-eating”) is the process of degradation and recycling of unnecessary and dysfunctional cellular components, including organelles, that plays an important role in cell energy homeostasis. Studies of autophagy as a compensatory mechanism required for cell survival during an energy crisis were initiated by experiments with nitrogen starvation in yeasts [1]. Since then, investigations of the roles and molecular players of this catabolic pathway in different eukaryotes continue to increase.
In plants, three major types of autophagy have been described: macroautophagy, microautophagy and mega-autophagy, or mega-autolysis [2–4]. Each of them is characterized by specific cytological features. Macroautophagy is the most universal and best studied process whose induction is accompanied by the formation of autophagosomes, vesicles enclosed by double-membranes encapsulating portions of cytoplasmic material (cargo) destined for lytic degradation [5]. After the delivery of the autophagosome to the central vacuole its outer membrane fuses with the tonoplast and its cargo, surrounded by the inner autophagosomal membrane called ‘autophagic body’, is released into the vacuole. Products of cargo cleavage by vacuolar hydrolases can be stored in the vacuole or be transported to the cytosol by permeases and used there to meet current metabolic demands [3]. The induction of macroautophagy was observed in cells of different tissues for a wide range of plant species [3, 4, 6–8]. Microautophagy occurs by means of direct engulfment of cytoplasmic material by the tonoplast membrane through the formation of invaginations or protrusions [9, 10]. Due to the lack of specific molecular markers, our knowledge of the mechanisms of microautophagy and its roles in plant catabolism is still restricted [9, 10]. The most extreme route of intracellular degradation in plants is mega-autophagy. The main cytological event in mega-autophagy is the disruption of the tonoplast and the release of vacuolar hydrolases into the cytosol which results in digestion of the total cell content, sometimes including the cell wall [2, 11]. Usually, this type of autophagy represents the final step of programmed cell death (PCD) induced as part of a morphogenetic process, e.g. xylogenesis, or under pathogen attack, e.g. during the hypersensitive response [3, 11, 12]. For plants, the concept of a genetically programmed mechanism leading to cell death via autolysis was proposed for the first time by Yuri V. Gamalei during his studies of tracheid differentiation in spruce roots [13, 14].
So-called constitutive, or basal, autophagy is responsible for low rate degradation of cell components under favorable conditions, representing a quality control system for long-lived proteins and organelles [3, 4, 15]. Almost all known abiotic stresses (nutrient starvation, hypoxia, oxidative stress, drought, salt and osmotic stresses) as well as pathogen attacks can induce the formation of multiple autophagosomes and cause so-called stress-induced autophagy [4, 6, 7, 12]. This process is associated with a substantial enhancement of autophagic flux, i.e. the rate of degradation of cell components from the start point of cargo engulfment to the end point of its degradation in the central lytic vacuole (so-called productive or complete autophagy) is above that observed during basal autophagy [16].
The initiation of autophagosomes formation and their assembly involve a set of proteins encoded by the ATG (AuTophaGy-related) genes. About 20 ATG-proteins required for different stages of autophagy in yeasts, animals and plants have been described in recent decades [17]. Current comparative genomics revealed general evolutional conservation of the ATG genes among all groups of eukaryotes [18, 19]. In contrast to animals and yeasts, in plants a large number of ATG-proteins are encoded by gene families (e.g. four ATG1 homologs, two homologs each of ATG13 and ATG14, and eight homologs of ATG8 were identified in the Arabidopsis thaliana genome while in yeast, all these genes are present as single copies); furthermore, there are also plant-specific molecular players of autophagy [5, 17, 20, 21]. Expression of ATG genes was found in all groups of algae with the exception of Rhodophyta, where canonical ATG proteins are missing [18].
In plants six major core ATG protein groups take part in autophagosome biogenesis: (1) the ATG1 kinase complex (ATG1, ATG13, ATG11, ATG101, VPS34, VPS15); (2) the transmembrane protein ATG9; (3) the class III phosphoinositide 3-kinase (PI3K) complex (ATG6, ATG14); (4) the ATG2-ATG18 complex; (5) the system of ATG8 lipidation; (6) the system of ATG12 conjugation [5, 17, 20]. The assembly of autophagosomes involves several steps: first, the cup-like pre-autophagosome structure forms, predominantly originating from ER subdomains (omegasomes); then, it detaches from the ER resulting in the formation of the double-membrane phagophore (also known as the isolation membrane) which expands and then bends into a spherical shape enclosing target particles (cargo) to be degraded, thus forming a typical autophagosome. The process of autophagy can be divided into several steps [17, 22]. (1) The initiation of autophagy occurs in response to the signals which trigger dephosphorylation of ATG13 and the activation of the initial ATG1/ATG13 complex. (2) The nucleation of a pre-autophagosome structure, enclosed in a double membrane, at the phagophore assembly site (PAS) results in the separation of a flat vesicle from the ER. The membranes of the nascent phagophore are decorated with phosphatidylinositol 3-phosphate (PtdIns3P) by the PI3K complex, which is highly important for the subsequent recruitment of various PtdIns3P-binding ATG proteins. The source of the phagophore membrane material was shown to represent specific ER subdomains which fuse with ATG9-containing vesicles detaching from the trans-Golgi network. (3) The phagophore expansion, i.e. the enlargement of the isolation membrane surrounding the cargo, is facilitated by the fusion with ATG9-containing vesicles and is assisted by the ATG2-ATG18 complex. The ATG8 lipidation system and the ATG12 conjugation system further decorate the phagophore membrane with ATG8 lipidated with phosphatidylethanolamine (ATG8-PE). (4) The next stage includes the maturation of the phagophore, the closure of its membrane, the transport of the autophagosome through the cytosol and its fusion with endosomes enriched by lipids and proteins required for future autophagosome fusion with the central lytic vacuole. (5) Finally, the fusion of the outer autophagosome membrane with the tonoplast results in cargo release into the vacuole and formation of the internal vacuolar vesicle, the autophagic body [17, 22].
Interestingly, contrarily to the single ATG8 gene in yeast, plant genomes contain multiple ATG8 orthologues [21], which are probably involved in recognition of different types of cargo during so-called selective autophagy. Apart from the key role in phagophore eхpansion and autophagosome maturation, the ATG8-PE conjugates also bind ATG8-interacting adaptor and receptor proteins responsible for cargo selection. The ATG8-interacting proteins usually contain the ATG8-interacting motif (AIM) or the ubiquitin-interacting motif (UIM) for ATG8 binding [23, 24]. Selective autophagy plays a crucial role in cell homeostasis; recent investigations suggest that in plants, degradation of cell components mostly occurs via selective mechanisms.
This review focuses on interactions between autophagy as a major catabolic pathway in eukaryotes, and the energy status of plant cells.
ENERGY STATUS OF PLANT CELLS AND POSSIBLE REASONS FOR ENERGY DEPLETION
Adenosine-5'-triphosphate (ATP) is known as ubiquitous energy currency and serves as energy source for various metabolic reactions inside the cell. The ATP molecule consists of a nitrogen-containing organic base (adenine), a sugar (ribose) and a string of three phosphate groups. The transfer of phosphoryl, pyrophosphoryl or adenyl groups from ATP provides energy for enzymatic reactions. Two phosphoryl end groups of ATP form macroergic bonds which can be sequentially hydrolyzed with the release of free energy (∆G0' is ca. –30.6 kJ/mol per break of each bond at standard conditions) and the formation of ADP or AMP, respectively. A majority of enzymes use ATP and ADP as magnesium chelates (MgATP and MgADP). The uncomplexed forms of both ATP and ADP may cause appreciable inhibition of many enzymes, especially kinases [25]. The role of ATP is not limited to short-term energy storage and transfer within the cell as it also functions as a substrate in nuclear acid biosynthesis, as allosteric cofactor of some enzymes and as biological hydrotrope to keep proteins soluble [26]. Recent studies revealed the existence of an extracellular ATP pool (eATP) in the apoplast. ATP can be released into the extracellular matrix via ATP-binding cassette (ABC) transporters and the PM-ANT1 (Plasma Membrane Adenine Nucleotide Translocator 1) nucleotide transporter, by active exocytosis or via mechanically wounded cell membranes. Apoplastic eATP is assumed to play an important signaling role in plant stress responses [27, 28].
The balance between processes of ATP generation and consumption determines the energy status of a plant cell. Anabolic reactions require high levels of activity of cytosolic enzymes which in turn depend on ATP supply. ATP levels are maintained mostly by ATP regeneration from ADP and inorganic phosphate (Pi) pools. Under normoxic conditions, the main part of the ATP pool is generated by ATP synthases localized in the inner membranes of the energy-producing organelles, chloroplasts and mitochondria [29]. The contribution of each organelle type to cytosolic and nuclear ATP concentrations in green plant cells in the dark and in the light was investigated by many research groups including the Laboratory of photosynthesis (currently Laboratory of molecular and ecological physiology) headed by Olga Aleksandrovna Semikhatova at the Komarov Botanical Institute of the Academy of Sciences of the USSR in the 1980s [30, 31]. The extreme complexity of experimental methods required to measure ATP concentrations in subcellular compartments remains one of challenging factors; another factor is the high rate of adenylate interconversions. Nowadays, the application of three modern detection methods, the improved method of rapid fractionation of protoplasts by membrane filtration [32], 31P-NMR spectroscopy [33] and generation of Arabidopsis sensor lines expressing the ATP biosensors ATeam1.03-nD/nA combined with specific inhibitors [34], allowed to achieve non-contradictory data on the ratios of adenylate levels and to measure their absolute concentrations. In several model plant systems, ATP concentrations in the cytosol were shown to vary within 1–2 mM [33, 34]. In different tissues of 10-days Arabidopsis seedlings, the concentrations of metabolically active chelated MgATP were between 0.75 ± 0.16 mM in root cells and above 1.4 mM in cotyledons.
Over their life span, plants experience fluctuations of their energy status which can be caused either by external reasons such as the daily cycle of light and darkness, short-term shading, or by environmental stresses like drought, flooding, soil salinity, low or high air temperatures, or nutrient deprivation. The energy deficit associated with stress causes transcriptional, translational, and metabolic changes termed ‘low energy syndrome’ (LES) [35, 36]. The roles of the two main cellular regulatory protein kinases, TOR and SnRK1, in the regulation of energy homeostasis, adaptive reprogramming of cell metabolism and stress responses will be discussed below.
ADENYLATE EQUILIBRIUM IN PLANT CELLS
In plant cells, the energy homeostasis is supported by continuous dynamic regulation of concentrations of adenine nucleotides AMP, ADP and ATP. In accordance with the theory of adenylate energy charge developed by Atkinson, the energy status can be estimated using the adenylate energy charge (AEC). This parameter refers to the relative saturation of the adenylate pool by phosphoanhydride bonds and is defined as ([ATP] + 1/2[ADP])/([ATP] + [ADP] + [AMP]) [37]. The value of the energy charge ranges from zero (when only AMP is present in solution) to 1 (when all AMP molecules have been converted to ATP). It was shown that the energy charge of mitochondria, chloroplasts and cytosol in the light after 5 min of dark-light transition is maintained at levels of ca. 0.70–0.94 [38, 39]. In plant cells, the key role in controlling the ratio of adenylates and governing their equilibrium is ascribed to the enzyme adenylate kinase (AK; ATP:AMP phosphotransferase, EC 2.7.4.3). AK catalyzes the reversible reaction of the formation of two ADP molecules from ATP and AMP. Taking into account the magnesium chelation by substrates and products, the reaction catalyzed by AK can be presented as follows: MgADP + ADP ↔ MgATP + AMP [25]. AK is responsible for the recycling of AMP and ADP formed in metabolic reactions energized by ATP, and has a great impact on the AEC [40]. In the Arabidopsis thaliana genome, eight genes encoding AK isoforms were identified. AK activity is distributed over four cell compartments, the cytosol, mitochondria, plastids and the nucleus [25]. In photosynthesizing leaf cells, pools of adenylate species are unequally distributed among compartments: approximately 45% are located in the plastid stroma, 46% in the cytosol-nuclei fraction, and 9% in mitochondria [39]. The cellular adenylate balance regulated by AK is an important buffering mechanism maintaining optimal ATP contents in subcellular compartments, preventing both overaccumulation and a critical drop in the levels of adenylates with macroergic bonds, i.e. ATP and ADP.
The question about the contribution of the two ATP-producing organelles, mitochondria and chloroplasts, to cytosolic ATP levels in the light and in the dark was debated for many decades. The current view is that the source of both bulk adenylates in the cytosol and of Mg ions needed for adenylate chelation is the chondriome [29, 39]. Cytosolic concentrations of MgATP both in the dark and in the light mainly depend on ATP produced in mitochondrial oxidative phosphorylation, not in chloroplasts [29, 41–44]. Although ATP is synthesized in the light also in chloroplasts, it is not exported to the cytosol; however, triose phosphates are exported in exchange with Pi, and malate in exchange with oxaloacetate, and these processes are important for the maintenance of energy balance in the light [29, 42]. In the light, malate formed in the chloroplasts is oxidized in mitochondrial respiration, thus utilizing the surplus of NADPH generated in chloroplasts that is critical for the maintenance of the stromal NADP+ pool and high rate of linear electron transport [43, 44]. Furthermore, mitochondrial malate oxidation provides ATP energy for sucrose synthesis in the cytosol [43, 44]. Chloroplasts do not import ATP from the cytosol and thus do not contribute to the decrease of its cytosolic levels either in the light or in darkness [44, 45].
Depending on the phosphorylation level and Mg chelation, adenine nucleotides can regulate a number of metabolic processes [25]. The MgATP/MgADP ratios in cell compartments reflect the capacity for the maintenance of anabolic reactions; the ratios of Mg2+-free ATP/ADP reflect the potential of adenylate export because only non-chelated adenine nucleotides are transported across cell membranes; the MgАTP/AMP ratios reflect the potential for allosteric regulation of enzymes [45]. Most MgATP-dependent enzymes are inhibited by MgАDP via the mechanism of competitive inhibition; thus their activity depends on the [MgАТP]/[MgАDP] ratio [45]. Furthermore, Mg2+-free AMP and/or ADP can act as allosteric regulators of a number of enzymes. Due to the fundamental role of Mg ions in the regulation of ATP-dependent enzymes, the actual energy charge of a cell depends on Mg2+-free adenylates, Mg2+-chelated adenylates and Mg2+ [25].
АК has an essential impact on plant cell catabolism as it maintains the dynamic regulation of the levels of AMP, the metabolite which is a highly sensitive marker of changes in the cell energy status. In metabolic reactions, the most common type of ATP hydrolysis is the transfer of one phosphoryl group resulting in the formation of ADP. Then, AMP can be formed in the reaction catalyzed by AK. A drop in the ATP levels by 10% can lead to a tenfold increase in AMP levels; therefore, multiple mechanisms of metabolic regulation rely on AMP concentrations.
SNRK1 KINASE AS A SENSOR OF THE ENERGY STATUS
The highly conserved protein kinase SnRK1 (Sucrose non-fermenting 1–related kinase 1) is one of the main regulators of plant metabolism, maintaining a balance between the levels of energy and activation of energy-consuming metabolic pathways associated with growth and stress responses. The orthologues of SnRK1, AMPK in mammals and SNF1 in yeast, function as sensors of cellular AMP concentrations and change their activity depending on the energy charge, which occurs via an allosteric mechanism [46]. In contrast, plant SnRK1 kinase is not allosterically regulated by AMP molecules; however, its activity correlates with the AMP/ATP or ADP/ATP ratios in the cell [47]. In response to an energy deficit, SnRK1 restores the energy balance by increasing mitochondrial ATP production, glycolysis and fatty acid oxidation on the one hand, and inhibiting ATP-consuming biosynthetic pathways on the other hand [47]. This can occur by means of direct phosphorylation of key enzymes of the metabolic pathway, for instance, hydroxymethyl glutarylCoA reductase or sucrose phosphate synthase, or by transcriptional regulation, e.g. of ATG genes [47–50].
SnRK1 kinase functions as a heterotrimeric complex consisting of a catalytic α-subunit and regulatory β- and βγ-subunits. Each type of subunit occurs in several isoforms. For Arabidopsis thaliana, six active combinations of subunits within a SnRK1 kinase complex have been described. In plant genomes, genes encoding three isoforms of the α-subunit (SnRK1α1/KIN10, SnRK1α2/KIN11 and SnRK1α3/KIN12), three isoforms of the β-subunit (SnRK1β1, SnRK1β2, SnRK1β3) and one isoform of the βγ-subunit (SnRK1βγ) were identified [51, 52]. The homolog of the γ-subunit of the mammalian AMPK kinase and yeast SNF1 kinase, SnRK1γ, has also been described in plants, but it is not part of the active SnRK1 complex and its functions are still unknown [52]. Subunits β and βγ are regulatory and include carbohydrate binding domains (CBM), which can potentially be responsible for kinase activity, localization and substrate specificity of the SnRK1 complex [51]. In the structure of βγ, a CBS (cystathionine β-synthase) domain was identified, which probably binds adenine nucleotides and determines conformation and activity of the kinase complex in response to changes of the energy charge. In cereals, SnRK1β-encoding genes remain inactive in most organs and are expressed only in seeds; the plant-specific β3 isoform (SnRK1β3) lacks the N-terminal region and CBM, but is still assembled into SnRK1 complexes [51]. The primary activation of the newly synthesized native SnRK1 proteins requires their phosphorylation at the threonine Thr175 (SnRK1α1) or Thr176 (SnRK1α2) amino acid residue in a T-loop (“loop activation”) of the catalytic domain, which is carried out by the SnRK-Activating Kinases 1 (SnAK1/GRIK1) and SnAK2/GRIK2. SnRK1 activated this way is further capable of autophosphorylation [49].
It has been suggested that the α-subunits can be phosphorylated and activated as free subunits and not only as a part of the SnRK1 complex, and can act independently of other subunits. Overexpression of the α-subunit at normal levels of expression of the remaining subunits leads to a high specific activity of SnRK1 in leaf cells of transgenic plants [51]. This leads to the conclusion that, in contrast to the γ-subunit-mediated activation under energy deficit which occurs in mammalian and yeast SnRK1 homologues AMPK and SNF1, plant SnRK1 is regulated by inhibition under energy surplus conditions. To date, it has been established that the activity of SnRK1 is inhibited by sugar phosphates, trehalose-6-phosphate (T6P), glucose-6-phosphate and glucose-1-phosphate [50]. The T6P levels correlate with the levels of sucrose available for metabolism, and thus T6P is an indicator of the metabolic status of the cell. It can be speculated that T6P binds directly to the catalytic subunits of SnRK1α and interferes with the interaction of SnAK kinases and the T-loop. Another disaccharide, maltose, formed in the hydrolysis of transient starch in leaves during the night, acts as an allosteric activator of SnRK1, although the exact binding site of maltose is still unknown [47, 53]. One of the mechanisms of suppressing the activity of the SnRK1 in the absence of stress is dephosphorylation of the catalytic α-subunit at the DUF581 domain (domain of unknown function 581) by protein phosphatases ABI1/PP2Cs and PP2CA. The phytohormone ABA inhibits these phosphatases and increases the activity of SnRK1 upon the onset of stressful conditions [53]. Hyperactivation of SnRK1-mediated signaling cascades during the development of a stress response is prevented by continuous sumoylation, ubiquitinylation, and subsequent proteasomal degradation of active SnRK1 complexes [53].
Studies of the regulation of SnRK1 by chloroplast and mitochondrial retrograde signals, which may be another way of integration of SnRK1 into plant energy metabolism, are of growing interest [51]. In Arabidopsis, subunits SnRK1α1/KIN10 and SnRK1α2/KIN11 were detected in the nucleus and in the cytosol. In the nucleus, SnRK1α1 regulates the expression of nuclear genes by interacting with transcription factor proteins, for example, bZIP63, which regulate groups of genes encoding shuttle carriers of organic acids in organelle membranes [49]. Several studies reported the occurrence of SnRK1α1 in chloroplasts [47, 51, 53]. It is not known whether SnRK1, or its ortholog AMPK, can be translocated into mitochondria; however, the role of AMPK in the regulation of mitochondrial functions and in the activation of autophagic degradation of damaged mitochondria (mitophagy) is extremely important. If it would be possible to prove whether part of the SnRK1 pool is localized in chloroplasts or mitochondria, many enigmatic aspects of SnRK1 function would have been explained.
REGULATION OF AUTOPHAGY THROUGH TOR AND SNRK1 SIGNALING PATHWAYS
Under conditions favorable for growth, autophagy (macroautophagy) is inhibited by the TOR (target of rapamycin) kinase complex, the main activator of anabolism and supressor of catabolism in eukaryotic cells. The TOR kinase complex comprises TOR, a highly conserved Ser/Thr kinase, and two regulatory proteins, RAPTOR (Regulatory-Associated Protein of TOR) and LST8 (lethal with SEC-thirteen protein 8) [47]. TOR regulates cell metabolism by the direct phosphorylation of multiple target proteins, for instance, a ribosomal kinase S6K, which results in the activation of protein translation; TOR also causes changes in the transcription profiles of genes related to metabolism, cell cycle and signal transduction [54]. TOR-activating factors include light, auxin and nutrient availability, especially the levels of glucose [46, 47, 55]. Nutrient depletion leads to inhibition of the TOR kinase complex and a decrease in the phosphorylation level of multiple TOR targets including ATG13 which in turn permits the assembly of the ATG1/ATG13 initiating complex and thus induces autophagy.
As a sensor of cellular energy status, SnRK1 is able to induce autophagy in response to a decrease in the levels of sugar-phosphates via TOR-dependent and TOR-independent pathways. SnRK1 can directly inhibit the TOR kinase complex by phosphorylation of its component RAPTOR [46]. SnRK1 also maintains hyperphosphorylation of ATG1 and enables the assembly of ATG1/ATG13, bypassing the inhibition by the TOR-kinase complex [56]. Under starvation, salt or osmotic stress SnRK1 activates autophagy via TOR inhibition, while under oxidative and ER-stress SnRK1 activates autophagy via the ATG1/ATG13 complex [5]. Apart from this, a recent study suggests that SnRK1 is able to phosphorylate ATG6 and to induce autophagy in TOR- and ATG1-independent manner under prolonged carbon starvation [56].
In mammals, TOR can be inactivated by metabolic signaling via amino acid availability required to support protein synthesis. Contrarily, in plants glucose is the major and possibly the only metabolite which directly regulates TOR [57]. Similarly, TOR inactivation by the lack of sulfur in Arabidopsis appears to be mediated by signals from glucose metabolism [58]. Although amino acids activate the TOR signaling pathway in plants leading to inhibition of respiration by targeting the expression of genes involved in amino acid catabolism, this mechanism is not involved in the regulation of autophagy [59]. As the activity of SnRK1 kinase in plants is regulated mainly by sugar phosphates and not by direct interaction with AMP or via the energy charge, it can be concluded that sugars are the key regulators of the TOR-SnRK1 module in plants [60]. Indeed, in plants as photo-autotrophic organisms, the inherent way of using the energy of sunlight is the biosynthesis of sugars, and sugar molecules serve as storage and transport forms of assimilated carbon. Therefore, studies of physiological roles of autophagy in plants should include its role in sink-source relations and the distribution of assimilates over the plant organism. Studies of autophagy in economically important crops can potentially exert great impact on the improvement of agricultural productivity and stress tolerance in crops [61].
Thus, two functional antagonists, TOR and SnRK1 kinases, play important regulatory roles in plant growth and stress responses, but the result of their action mainly depends on conditions plants have to cope with. Our knowledge about the molecular players of SnRK1 and TOR-mediated signaling cascades and the mechanisms of their crosstalk in plants permanently expands and deepens. To maintain high productivity in plants subjected to various stresses, simultaneous activation of growth and defense programmes has to be achieved. Fine tuning of the TOR-SnRK1 regulatory module will be a promising tool for the optimization of crop productivity.
INTEGRATION OF AUTOPHAGY IN PLANT CELL METABOLISM
As the major catabolic programme in eukaryotes, autophagy is intimately integrated in plant metabolism as confirmed i.a. by metabolomics data [62], and is itself subjected to metabolic regulation via the TOR kinase complex and probably also other thus far unknown mechanisms [57]. While constitutive autophagy removes damaged or unwanted cellular components in a highly selective manner (as exemplified, for instance, by the removal of the flagellin receptor kinase FLS2 from the plasma membrane of Arabidopsis cells [12]), stress-induced bulk autophagy mediates massive lysis of many cell parts including storage compounds. The induction of autophagy above its constitutive level can occur also in the absence of stress, for instance, during seed germination which requires a rapid and massive utilization of storage compounds providing energy and metabolites for biosynthetic processes [63, 64]. Similarly, ‘nocturnal autophagy’ is induced in leaf cells during the night and participates in the degradation of transitory starch in lytic vacuoles [65]. In such situations, autophagic degradation of proteins, lipids, starch and RNA occurs in plant cells; accordingly, the presence of proteases and lipases, as well as amylases and nucleases, has been reported in plant autophagosomes and lytic vacuoles [66, 67]. Thus, autophagy has an essential impact on the cell energy status providing metabolites for the synthesis of new polymers and structures as well as for an increase in ATP production.
Autophagy and Protein Metabolism
Several studies show that, compared to other polymers, proteins are subjected to oxidative modifications at the highest extent; this has been shown for instance for cells exposed to singlet oxygen [68]. The role of autophagy in the clearance of damaged proteins from cells increases several fold during oxidative stress [3]. Compared to proteasomal degradation, autophagy provides a more energy-requiring but much more effective mechanism of removal of damaged proteins: the whole protein complexes including proteasomes, protein aggregates and even organelles or their parts can be degraded at once [15]. The resulting amino acids, after deamination, enter the Krebs cycle and thus ensure ATP production in mitochondria [69].
Autophagic Degradation of Nucleic Acids
Ribophagy is a specialized type of autophagy targeting ribosomes for their hydrolysis by nucleases in the vacuole. Selective ribophagy as a mechanism of removal of damaged ribosomes has been documented in yeast and animal cells but thus far not in plants [15]. At the same time, autophagic degradation of ribosomes during stress-induced ‘bulk’ autophagy (currently considered as a non-selective process) has been observed in cells of plants and algae [67]. RNA hydrolysis in the vacuole represents the main source of nucleosides and free ribonucleotides in cells; furthermore, as deoxyribonucleotides are synthesized from ribonucleotides, autophagic degradation of ribosomes is of utmost importance for nucleotide homeostasis in eukaryotes [15, 28]. In animal cells, autophagy of DNA (DNautophagy), in particular the lysosomal degradation of mitochondrial DNA, has been described [67]. Apart from this, DNA damage in animal cells leads to activation of a complex of protective reactions known as ‘DNA damage response’ which includes autophagy, to provide metabolic precursors of ATP and DNA synthesis to ensure optimal repair of DNA [70]. As the DNA damage response was also observed in plants [71], we can speculate that autophagy might play a similar role there.
Autophagy and Hydrolysis of Leaf Transitory Starch in the Night
In leaf mesophyll cells, the export of maltose and glucose representing breakdown products of transitory starch from chloroplasts to the cytosol in the night ensures the flow of photoassimilates to sink organs and tissues, and also sustains the metabolism of mesophyll cells. Surprisingly, in addition to nocturnal hydrolysis of starch directly in chloroplasts, a part of it (in form of small starch granule-like structures, SSGL) is transported to vacuoles and degraded there; this process relies on autophagy and on microtubule functions [65]. In tobacco leaves, inhibition of the microtubule cytoskeleton caused an increase of starch levels which led to induction of partial chlorophagy: pieces of chloroplasts containing starch granules were observed in the vacuoles [65]. Thus, in the night, autophagy serves the maintenance of metabolism in sink organs and tissues via transport of products of hydrolysis of starch synthesized in leaves during photosynthesis, and also the maintenance of energy metabolism of mesophyll cells of source organs, the leaves. It can be speculated that the levels of autophagy and starch hydrolysis are related via SnRK1regulatory kinase: one of the starch breakdown products, maltose, can activate an SnRK1 isoform presumably localized in chloroplasts [52]. How the putative signals from the chloroplastic SnRK1 are transduced and sensed in the cytosol remains elusive.
The Role of Autophagy in Metabolism of Lipids in Plants and Algae
In plant cells autophagy takes part both in lipid biosynthesis and catabolism. Plants cells contain two main pools of lipids, membrane lipids and neutral lipids (triacylglycerols, TAG) accumulating in a specialized type of organelles, the lipid droplets (LDs). In plants, contrarily to yeast and animals, participation of autophagy in the metabolism of TAG has been shown only recently [3]. Similar to animal cells, in mature leaves of Arabidopsis constitutive autophagy is required for TAG biosynthesis from membrane lipids and their accumulation in LDs [72]. In green plant cells, lipid biosynthesis is compartmentalized in chloroplasts and at the ER membrane [72, 73]. The substrate for TAG biosynthesis are fatty acids formed in the vacuole after autophagic degradation of membrane lipids of those organelles whose membranes originate from the ER (including the autophagosomes themselves). The inner chloroplast membranes, which consist of lipids synthesized in the chloroplasts, normally are not subjected to autophagic degradation and thus do not serve as substrates for TAG biosynthesis [72, 73]. Furthermore, starvation-induced autophagic degradation of LDs (lipophagy) in Arabidopsis occurs via microautophagy without formation of autophagosomes, similar to yeast but contrarily to animals [72]. Microautophagy results in production of fatty acids from TAG in the vacuole which are further subjected to beta-oxidation. Notably, starvation-induced lipophagy in plant cells can occur simultaneously with the biogenesis of TAG-containing LDs via constitutive autophagy [72].
In the microalga Chlamydomonas reinhardtii, TAG biosynthesis and LD biogenesis also depend on autophagy [74]; however, contrarily to Arabidopsis, an increased formation of LDs is observed during autophagy induced by deficiency of macronutrients. Under conditions of phosphorus or, especially, nitrogen deficiency, Chlamydomonas cells undergo global restructuring and switch to increased accumulation of carbon in the form of TAG; autophagy plays an important role in this process [74]. Transition from heterotrophy to autotrophy in the microalga Auxenochlorella protothecoides caused degradation of LDs via microautophagy which was accompanied by chloroplast biogenesis [75]. In general, autophagy in microalgae seems to serve primarily the interconversions of chloroplast membrane lipids and TAG, both under starvation conditions and during transition from hetero- to autotrophic growth (which in the first stages is also accompanied with carbon starvation). Both in plants and in microalgae, lipolysis in LDs might represent a necessary step to diminish the size of LDs, thus facilitating their sequestration in the vacuole via microautophagy [72, 75]. The functions of autophagy in lipid metabolism—both in conversion of membrane lipids to energy-rich storage TAG and in their catabolism in the vacuole producing fatty acids, followed by beta-oxidation and production of energy—represent an important contribution to the regulation of the energy status of plant cells which remains open to further investigations.
Autophagic Degradation of Storage Compounds during Seed Germination
Autophagy has important functions in formation and maturation of seeds [76], including transport of storage compounds such as proteins to storage vacuoles [77]. However, to provide germinating seeds with energy and substrates for biosynthesis, hydrolysis of storage compounds is of utmost importance, and autophagy plays a role in this process. In cotyledons of germinating mungo bean (Vigna mungo), microautophagy participates in the transport of protein bodies to the vacuoles; these bodies contain lytic enzymes, acid hydrolases with proteolytic, lipolytic, phosphatase and ribonuclease activities [63]; it also takes part in degradation of storage starch [64]. In seeds of castor bean, cytological evidence has been obtained for autophagic degradation of storage proteins [78]. During germination of oil-storing seeds, for instance in Arabidopsis, the utilization of TAG starts with their lipolysis in LDs (also called oleosomes) and ended with the beta-oxidation of the formed fatty acids in glyoxysomes. For this process, autophagic degradation of damaged glyoxysomes is highly important as a quality control mechanism [79]. However, there might be also other roles for autophagy in the degradation of LDs in germinating seeds. Hydrolysis of TAG requires ubiquitinylation of oleosome-coating proteins, oleosines; however, ubiquitinylation can serve as a signal for not only proteasomal but also autophagic degradation. The recently discovered recognition of a new class of selective adaptor and receptor proteins with UIM motifs by ATG8 proteins is able to mediate interactions of oleosines with ATG8 in vitro [23]. It can be speculated that a similar mechanism might mediate the capture of LDs by autophagosomes in germinating seeds [73].
MITOPHAGY AND CHLOROPHAGY AS ROUTES OF UTILIZATION OF ENERGY-PRODUCING ORGANELLES
In plant cells the energy and redox homeostasis is supported by the coordinated function of chloroplasts and mitochondria. These energy-producing organelles are sources of dangerous reactive oxygen species (ROS) whose overproduction under stress conditions can cause the oxidative burst and cell death [80, 81]. Autophagy-mediated selective degradation and utilization of damaged plastids and mitochondria (named chlorophagy and mitophagy, respectively) represents a quality control mechanism for these organelles [80]. Although peroxisomes cannot be referred to as energy-producing organelles, they are involved in photorespiration and the glyoxylate cycle both of which are indispensable under stress conditions or during seed germination. Thus, they function in close coordination with chloroplasts and mitochondria. Furthermore, peroxisomes detoxify ROS and are subjected to high extents of oxidative damage. Therefore, autophagy of peroxisomes (pexophagy) is an important quality control mechanism for maintaining energy and redox homeostasis in plant cells [82].
Thus far, a unique plant-specific process of selective recycling of chloroplasts, chlorophagy, remains largely unexplored [23]. Two types, piecemeal chlorophagy and entire chlorophagy, have been described. During senescence or in response to nutrient (especially nitrogen) starvation, chloroplasts in Arabidopsis leaves form protrusions called stromules. Formation of stromules is suggested to depend on ATG5 and ATG8 and is accompanied by detachment of Rubisco-containing bodies (RCBs) [23]. Recently the CHMP1 (Charged Multivesicular Body 1) protein, a component of the endosomal sorting complex ESCRT, was shown to mediate detachment of RCBs vesicles and their delivery to the vacuole, as well as phagophore closure around the plastidial cargo [23]. Another type of piecemeal chlorophagy is characterized by the formation of SSGL bodies during nocturnal starch degradation. Other chloroplast-derived vesicles include ATG8-interacting protein bodies (ATI-bodies) formed during carbon starvation, e.g. under prolonged darkening of Arabidopsis seedlings. The membranes of ATI-bodies carry the chlorophagy receptors ATI1 and ATI2 (ATG8-Interacting Protein 1 and 2) able to bind stromal, thylakoid and outer chloroplast membrane proteins as ligands [23, 83].
Autophagic degradation of entire chloroplasts can be induced in response to specific damaging agents. In leaves exposed to high light or ultraviolet B (UVB) radiation, ROS accumulation activates the selective elimination of chloroplasts via ATG5- and ATG7-dependent mechanisms [84]. Removal of broken or damaged chloroplasts can occur simultaneously and independently via autophagy and ubiquitin-proteasome pathways [85]. Entire chlorophagy, accompanied by piecemeal chlorophagy and formation of RCBs, occurs also in senescing leaves subjected to extended dark treatment [85].
It remains to be determined which types of chloroplast damage elicit specific types of chlorophagy, and whether autophagic degradation of chloroplasts is controlled by the metabolic status of the cells. Recently, an additional way of chloroplast degradation specifically activated by internal enhancement of singlet oxygen production has been described. Chloroplasts damaged by singlet oxygen first undergo ubiquitinylation by cytosolic Plant U-Box 4 (PUB4) E3 ubiquitin ligase [86], and then are subjected to degradation via microautophagy [10]. Macroautophagy is not involved in this process since this type of chloroplast degradation is not blocked in ATG5 and ATG7 gene knockouts lacking the key components of autophagosome assembly [10]. As the generation of singlet oxygen in chloroplasts occurs frequently under high light [81], it is possible that microautophagy plays an essential role in maintaining a pool of functional chloroplasts in photosynthesizing cells.
The selective autophagy of mitochondria (mitophagy) represents another fundamental process of homeostasis regulation in eukaryotic cells. The chondriome in eukaryotic cells forms a complex dynamic network continuously changing the size and topology of mitochondria by mitochondrial fission and fusion processes [80]. In animal cells and yeast mitochondrial dynamics are closely linked to mitophagy. Multiple molecular agents mediating stress-induced mitophagy were identified [87, 88]. Weak defects in mitochondria trigger their fusion with healthy ones leading to the ‘dilution’ of damage and activating repair processes. Severe defects accumulating under strong stress promote the segregation of impaired small mitochondria by fission from their mother organelles, and their degradation by autophagy. When the strength of stress increases and the repair of damaged mitochondria becomes impossible, even large mitochondria are sequestered into autophagosomes [87]. Several outer mitochondrial membrane proteins responsible for the fission/fusion dynamics have been identified as mitophagy receptors [87].
The progress of mitophagy research in plants lags behind studies on yeast and animal cells. Strikingly, the mechanisms of autophagic degradation of mitochondria remain even less studied than those of chlorophagy [23]. Autophagosome-like structures containing mitochondria (mitophagosomes) were detected on electron micrographs in a wide range of plants during tissue differentiation and under stress [88]. In plant vegetative cells, mitochondria are usually round and do not form giant tubular structures although a recent study has shown that after exposure to UV-B, they can undergo fragmentation preceding mitophagic degradation similar to mammalian mitochondria [84]. Plant mitophagy is induced by uncoupling ionophores [88, 89]; the loss of membrane potential, similar to the situation in cells of other multicellular eukaryotes, serves as a marker of mitochondrial damage and triggers selective mitophagy [88]. One of the molecular regulators of mitochondrial fusion, FRIENDLY MITOCHONDRIA (FMT), a protein of the CLUSTERED MITOCHONDRIA superfamily, accumulates on the surface of damaged mitochondria and co-localizes with ATG8, suggesting a role as a receptor/adaptor complex in the regulation of mitophagy in plants [89]. During seedling de-etiolation, the pool of mitochondria in cotyledon cells undergoes renewal by the mechanism of FMT-mediated mitophagy in order to reset the mitochondrial metabolic network for adaptation to illumination and release of substrates necessary for biogenesis of chloroplasts [89]. Cardiolipin synthase and E3-Ub ligase family proteins mediating lipid-dependent and ubiquitin-dependent mitophagy, respectively, in mammals, were identified as regulators of mitochondria biogenesis in plants, but their involvement in mitophagy has not been confirmed [80]. Oxidative stress caused by simultaneous inhibition of cytochrome and alternative respiratory pathways in mitochondria induced high levels of autophagic degradation including mitophagy in wheat root cells [90]. Of all plant ATG proteins, participation in mitophagy during senescence or carbon starvation was demonstrated for ATG11, ATG7, ATG8, ATG10 and ATG101 [80].
Summing up, mitophagy and chlorophagy help to solve one of the key problems of energy homeostasis, namely the maintenance of healthy and functional “energy-producing stations”. Under stress conditions, degradation of damaged organelles eliminates potential intracellular sites of excessive ROS generation, thus protecting the antioxidant systems from overload [6] and also preventing the release of toxic products of redox reactions from mitochondria. Furthermore, it can be speculated that, similar to the participation of mitophagy in the elimination of mitochondrial DNA in animal cells, autophagy in plants is also involved in preventing the genetic degradation of the mitochondrial population caused by the accumulation of somatic mutations in mitochondrial DNA [91].
ION HOMEOSTASIS IN THE REGULATION OF AUTOPHAGY: POTASSIUM AND MAGNESIUM
ATP synthesis in chloroplasts and mitochondria relies on the difference between the proton electrochemical potentials on both sides of the coupling membrane. At the same time, exchange of ions and solutes between the cytosol and the apoplast as well as between the cytosol and the organelles is energized by a difference in proton chemical potential created at the expense of ATP. In both cases, the built-up of a proton gradient is only possible when the transfer of protons is balanced electrically. Various ion fluxes can contribute to this balance, but a special role is played by potassium and magnesium. In chloroplasts, fluxes of magnesium ions balance the acidification of the lumen in the light and are directed from the thylakoid lumen to the stroma where Mg2+ fulfils an important function in RuBisCO activation [92]. Apart from this, as discussed above, Mg2+ ions are indispensable for ATP-consuming biochemical reactions occurring in the stroma. Potassium fluxes balance proton transport between the cytosol and chloroplasts or mitochondria as well as across the plasma membrane, the tonoplast and other acid endomembrane compartments [93–96]. Thus, the levels of K+ are related both to ATP syntheses in chloroplasts and mitochondria and to ATP hydrolysis during the acidification of the apoplast, the vacuole and other compartments.
It is important that the activities of the plasma membrane H+-ATPase and of the type I H+-pyrophosphatase are regulated by K+ [94]. At the same time, cytosolic homeostasis of K+ is required for maintenance of the activity of a number of cytosolic enzymes [97]. In Arabidopsis, potassium deficiency caused inhibition of over 50 enzymes including those participating in glycolysis and in nitrogen assimilation [98]. This led to the suggestion that the cytosolic levels of K+ might function as “metabolic switch” between anabolism and catabolism [97]. In mammals, cytosolic K+ inactivates caspases while the loss of K+ from the cells triggers PCD; similarly, in plants, leakage of K+ from root cells via outwardly-rectifying potassium channels GORK during salt stress triggers PCD [99] and autophagy [100]. However, the underlying mechanisms in plant cells remain elusive. As cellular K+ levels are intimately linked to energy metabolism and the energy status of plant cells, it can be speculated that a decrease in cellular K+ might activate SnRK1, as transmembrane K+ gradients are needed for the creation of proton gradients and ATP synthesis in chloroplasts and mitochondria. Another mechanism can include the effects of potassium deficiency on glycolytic enzymes [97]. The fact that potassium deficit inhibits glycolysis could be explained by the assumption that a decrease in the availability of pyruvate for mitochondrial respiration leads to a drop in cellular ATP levels. However, this hypothesis relating cellular K+ levels to the regulation of autophagy via energy metabolism still requires experimental proof.
DUAL ROLE OF AUTOPHAGY UNDER STRESS
Under suboptimal conditions, cells switch metabolism to an energy saving mode and ensure the release of low molecular weight metabolites via hydrolysis of storage compounds as well as the utilization of unnecessary components; these metabolites are then used to produce the lacking energy in order to maintain critical biosynthetic pathways. In most cases, stress-induced autophagy serves as the mechanism restoring energy supply and plays a cytoprotective role preventing the onset of PCD [101–103]. However, some not yet identified factors can elicit a special form of autophagy, mega-autophagy, or mega-autolysis, representing the final stage of vacuolar PCD [3, 11]. This process involves the aggregation of organelles, the inclusion of organelles into large vesicles, an increase of the volume of the central vacuole and rupture of the tonoplast leading to acidification of the cytosol and release of vacuolar processing enzymes (VPE), cysteine proteases with caspase-like activity which in turn activate other caspase-like enzymes [2, 101, 104, 105]. One of the reasons for the rupture of the tonoplast might be an improper coordination of autophagy with the osmotic adjustment of cell compartments [6]. The developing proteolytic cascade rapidly destroys the protoplast and even the cell wall. Vacuolar cell death has been linked to the induction of various developmental programs such as xylem differentiation, formation of aerenchyma in roots during hypoxia, change of leaf morphology as well as during the hypersensitive response or during leaf senescence [3, 11].
Autophagy is an indispensable component of the hypersensitive response where PCD develops in the course of the plant immune response to invasion of necrotrophic, hemibiotrophic or biotrophic pathogens [106]. Notably, the activation of hypersensitive response did not occur in the absence of peroxisomal catalase [107]. Studies with tobacco BY-2 suspension culture cells showed that the presence of catalase in peroxisomes is required also for the induction of PCD in the absence of pathogens, and that pexophagy as the mechanism of degradation of catalase might be required for PCD induction [108].
Recently, a direct relationship between autophagy and induction of PCD under nutrient deficiency has been demonstrated. During carbon starvation of tobacco BY-2 suspension culture cells, translocation of the precursor of the vacuolar processing enzyme StVPE1 to the vacuole was shown; for the first time, it was proven that VPE is transported via the autophagic route in ER-derived vesicles coated with StATG8IL protein fused to the RFP fluorescent marker [109]. Bioinformatic analysis of VPE expressed in vegetative tissues revealed ATG8-interacting motifs, pointing at a strict selectivity of the translocation of these enzymes. Thus, autophagy induced in response to energy deficiency in cells is able to directly activate PCD [109]. Yet, the dual role of autophagy in the determination of cell destiny during an energy crisis remains an important and understudied issue to be investigated in the future.
CONCLUSION
During the last decades, plenty of regulators involved in the switch between anabolic and catabolic processes in plants were successfully identified for many conditions. Key molecules influencing the energy status of plant cells have been revealed, and our knowledge about the regulation of autophagy in plants by the protein kinase complexes SnRK1 and TOR has progressed enormously. The realization that autophagy-related proteins are involved in the vesicular delivery of the precursors of vacuolar processing enzymes required for the execution of vacuolar type of PCD into the lytic vacuole was of particular importance [109]. At the same time, many aspects of the switch still remain unclear, e.g., the subcellular localization of the central protein kinase complexes SnRK1 and TOR in plant cells. The existence of a threshold in the cell energy charge below which the role of autophagic degradation switches from cytoprotection to one of the stages of cell death, also remains debatable. The important role of magnesium ions in cellular energy metabolism raises the question about their possible role in the regulation of autophagy and the SnRK1/TOR module in plants. Due to the energy expenses for the assembly of autophagosomes, their cytoskeleton-mediated transport and fusion with the tonoplast, autophagy itself is a highly energy-demanding process. Thus, it is important to understand how it is fueled. Furthermore, the question to what extent bulk autophagy really is non-selective has yet to be answered. Nutrient starvation triggers massive autophagic degradation of various cell components simultaneously. This type of autophagy is considered non-selective; indeed, selectivity of authophagy under these conditions would require additional energy expense [3]. However, in sucrose-starved tobacco cells the dynamics of autophagic degradation differed depending on the type of degraded organelles [110]. How is the degradation of each component regulated, and are specific receptors involved in such ‘non-selective’ autophagy? These questions await further investigations.
REFERENCES
Tsukada, M. and Ohsumi, Y., Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae, FEBS Lett., 1993, vol. 333, p. 169. https://doi.org/10.1016/0014-5793(93)80398-e
van Doorn, W.G. and Papini, A., Ultrastructure of autophagy in plant cells: a review, Autophagy, 2013, vol. 9, p. 1922. https://doi.org/10.4161/auto.2627
Marshall, R.S. and Vierstra, R.D., Autophagy: the master of bulk and selective recycling, Annu. Rev. Plant Biol., 2018, vol. 69, p. 173. https://doi.org/10.1146/annurev-arplant-042817-040606
Su, T., Li, X., Yang, M., Shao, Q., Zhao, Y., Ma, C., and Wang, P., Autophagy: an intracellular degradation pathway regulating plant survival and stress response, Front. Plant Sci., 2020, vol. 11, p. 164. https://doi.org/10.3389/fpls.2020.00164
Soto-Burgos, J., Zhuang, X., Jiang, L., and Bassham, D.C., Dynamics of autophagosome formation, Plant Physiol., 2018, vol. 176, p. 219. https://doi.org/10.1104/pp.17.01236
Signorelli, S., Tarkowski, Ł.P., van den Ende, W., and Bassham, D.C., Linking autophagy to abiotic and biotic stress responses, Trends Plant Sci., 2019, vol. 24, p. 413. https://doi.org/10.1016/j.tplants.2019.02.001
Chen, H., Dong, J., and Wang, T., Autophagy in plant abiotic stress management, Int. J. Mol. Sci., 2021, vol. 22, p. 4075. https://doi.org/10.3390/ijms22084075
Sirko, A. and Masclaux-Daubresse, C., Advances in plant autophagy, Cells, 2021, vol. 10, p. 194. https://doi.org/10.3390/cells10010194
Sieńko, K., Poormassalehgoo, A., Yamada, K., and Goto-Yamada, S., Microautophagy in plants: consideration of its molecular mechanism, Cells, 2020, vol. 9, p. 887. https://doi.org/10.3390/cells9040887
Lemke, M.D., Fisher, K.E., Kozlowska, M.A., Tano, D.W., and Woodson, J.D., The core autophagy machinery is not required for chloroplast singlet oxygen-mediated cell death in the Arabidopsis thaliana plastid ferrochelatase two mutant, BMC Plant Biol., 2021, vol. 21, p. 342. https://doi.org/10.1186/s12870-021-03119-x
Hatsugai, N., Yamada, K., Goto-Yamada, S., and Hara-Nishimura, I., Vacuolar processing enzyme in plant programmed cell death, Front. Plant Sci., 2015, vol. 6, p. 234. https://doi.org/10.3389/fpls.2015.00234
Dobryakova, K.S. and Voitsekhovskaya, O.V., Molecular aspects of basic innate immunity in Hordeum vulgare L., Ekol. Genet., 2020, vol. 18, p. 273. https://doi.org/10.17816/ecogen18648
Gamalei, Yu.V., Differentiation of tracheal elements of protoxylem of Picea abies L. (Karst.): electron microscope analysis, Cand. Sci. (Biol.) Dissertation, Leningrad: Komarov Bot. Inst., Acad. Sci. USSR, 1967.
Gamalei, Yu.V., Autolysis in differentiating tracheids, Tsitologiya, 1971, vol. 13, p. 278.
Yoon, S.H. and Chung, T., Protein and RNA quality control by autophagy in plant cells, Mol. Cells, 2019, vol. 42, p. 285. https://doi.org/10.14348/molcells.2019.0011
Klionsky, D.J., Abdel-Aziz, A.K., Abdelfatah, S., Abdellatif, M., Abdoli, A., Abel, S., Abeliovich, H., Abildgaard, M.H., Abudu, Y.P., Acevedo-Arozena, A., Adamopoulos, I.E., Adeli, K., Adolph, T.E., Adornetto, A., Aflaki, E., et al., Guidelines for the use and interpretation of assays for monitoring autophagy, Autophagy, 2021, vol. 17, p. 1. https://doi.org/10.1080/15548627.2020.1797280
Nakatogawa, H., Mechanisms governing autophagosome biogenesis, Nat. Rev. Mol. Cell Biol., 2020, vol. 21, p. 439. https://doi.org/10.1038/s41580-020-0241-0
Shemi, A., Ben-Dor, S., and Vardi, A., Elucidating the composition and conservation of the autophagy pathway in photosynthetic eukaryotes, Autophagy, 2015, vol. 11, p. 701. https://doi.org/10.1080/15548627.2015.1034407
Yoshimoto, K. and Ohsumi, Y., Unveiling the molecular mechanisms of plant autophagy – from autophagosomes to vacuoles in plants, Plant Cell Physiol., 2018, vol. 59, p. 1337. https://doi.org/10.1093/pcp/pcy112
Lai, L.T.F., Ye, H., Zhang, W., Jiang, L., and Lau, W.C.Y., Structural biology and electron microscopy of the autophagy molecular machinery, Cells, 2019, vol. 8, p. 1627. https://doi.org/10.3390/cells8121627
Bu, F., Yang, M., Guo, X., Huang, W., and Chen, L., Multiple functions of ATG8 family proteins in plant autophagy, Front. Cell. Dev. Biol., 2020, vol. 8, p. 466. https://doi.org/10.3389/fcell.2020.00466
Gomez, E.R., Joubès, J., Valentin, N., Batoko, H., Satiat-Jeunemaître, B., and Bernard, A., Lipids in membrane dynamics during autophagy in plants, J. Exp. Bot., 2018, vol. 69, p. 1287. https://doi.org/10.1093/jxb/erx392
Stephani, M. and Dagdas, Y., Plant selective autophagy—still an uncharted territory with a lot of hidden gems, J. Mol. Biol., 2019, vol. 423, p. 63. https://doi.org/10.1016/j.jmb.2019.06.028
Marshall, R.S., Hua, Z., Mali, S., McLoughlin, F., and Vierstra, R.D., ATG8-binding UIM proteins define a new class of autophagy adaptors and receptors, Cell, 2019, vol. 177, p. 766. https://doi.org/10.1016/j.cell.2019.02.009
Kleczkowski, L.A. and Igamberdiev, A.U., Magnesium signaling in plants, Int. J. Mol. Sci., 2021, vol. 22, p. 1159. https://doi.org/10.3390/ijms22031159
Patel, A., Malinovska, L., Saha, S., Wang, J., Alberti, S., Krishnan, Y., and Hyman, A.A., ATP as a biological hydrotrope, Science, 2017, vol. 356, p. 753. https://doi.org/10.1126/science.aaf6846
Pietrowska-Borek, M., Dobrogojski, J., Sobieszczuk-Nowicka, E., and Borek, S., New insight into plant signaling: extracellular ATP and uncommon nucleotides, Cells, 2020, vol. 9, p. 345. https://doi.org/10.3390/cells9020345
Witte, C.P. and Herde, M., Nucleotide metabolism in plants, Plant Physiol., 2020, vol. 182, p. 63. https://doi.org/10.1104/pp.19.00955
Gardeström, P. and Igamberdiev, A.U., The origin of cytosolic ATP in photosynthetic cells, Plant Physiol., 2016, vol. 157, p. 367. https://doi.org/10.1111/ppl.12455
Semikhatova, O.A., The role of respiration research in development of the theory of photosynthetic productivity of plants, Bot. Zh., 1982, vol. 67, p. 1025.
Semikhatova, O.A., Energy links between chloroplasts and mitochondria in the dark, Fiziol. Rast., 1992, vol. 39, p. 606.
Igamberdiev, A.U., Bykova, N.V., Lea, P.J., and Gardeström, P., The role of photorespiration in redox and energy balance of photosynthetic plant cells: a study with a barley mutant deficient in glycine decarboxylase, Physiol. Plant., 2001, vol. 111, p. 427. https://doi.org/10.1034/j.1399-3054.2001.1110402.x
Gout, E., Rébeillé, F., Douce, R., and Bligny, R., Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: unravelling the role of Mg2+ in cell respiration, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, p. 4560. https://doi.org/10.1073/pnas.1406251111
De Col, V., Fuchs, P., Nietzel, T., Elsässer, M., Voon, C.P., Candeo, A., Seeliger, I., Fricker, M.D., Grefen, C., Møller, I.M., Bassi, A., Lim, B.L., Zancani, M., Meyer, A.J., Costa, A., Wagner, S., and Schwarzländer, M., ATP sensing in living plant cells reveals tissue gradients and stress dynamics of energy physiology, eLife, 2017, vol. 18, p. e26770. https://doi.org/10.7554/eLife.26770
Baena-González, E. and Sheen, J., Convergent energy and stress signaling, Plant Sci., 2008, vol. 13, p. 474. https://doi.org/10.1016/j.tplants.2008.06.006
Tomé, F., Nägele, T., Adamo, M., Garg, A., Marco-Llorca, C., Nukarinen, E., Pedrotti, L., Peviani, A., Simeunovic, A., Tatkiewicz, A., Tomar, M., and Gamm, M., The low energy signaling network, Front. Plant Sci., 2014, vol. 5, p. 353. https://doi.org/10.3389/fpls.2014.00353
Atkinson, D.E., The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers, Biochemistry, 1968, vol. 7, p. 4030. https://doi.org/10.1021/bi00851a033
Hampp, R., Goller, M., and Ziegler, H., Adenylate levels, energy charge, and phosphorylation potential during dark-light and light-dark transition in chloroplasts, mitochondria, and cytosol of mesophyll protoplasts from Avena sativa L., Plant Physiol. 1982, vol. 69, p. 448. https://doi.org/10.1104/pp.69.2.448
Lange, P.R., Geserick, C., Tischendorf, G., and Zrenner, R., Functions of chloroplastic adenylate kinases in Arabidopsis, Plant Physiol., 2008, vol. 146, p. 492. https://doi.org/10.1104/pp.107.114702
Igamberdiev, A.U. and Kleczkowski, L.A., Equilibration of adenylates in the mitochondrial intermembrane space maintains respiration and regulates cytosolic metabolism, J. Exp. Bot., 2006, vol. 57, p. 2133. https://doi.org/10.1093/jxb/erl006
Liang, C., Zhang, Y., Cheng, S., Osorio, S., Sun, Y., Fernie, A.R., Cheung, C.Y., and Lim, B.L., Impacts of high ATP supply from chloroplasts and mitochondria on the leaf metabolism of Arabidopsis thaliana, Front. Plant Sci., 2015, vol. 6, p. 922. https://doi.org/10.3389/fpls.2015.00922
Igamberdiev, A.U. and Kleczkowski, L.A., Optimization of ATP synthase function in mitochondria and chloroplasts via the adenylate kinase equilibrium, Front. Plant Sci., 2015, vol. 6, p. 10. https://doi.org/10.3389/fpls.2015.00010
Kroemer, S. and Heldt, H.W., On the role of mitochondrial oxidative phosphorylation in photosynthesis metabolism as studied by the effect of oligomycin on photosynthesis in protoplasts and leaves of barley (Hordeum vulgare), Plant Physiol., 1991, vol. 95, p. 1270. https://doi.org/10.1104/pp.95.4.1270
Voon, C.P., Guan, X., Sun, Y., Sahu, A., Chan, M.N., Gardeström, P., Wagner, S., Fuchs, P., Nietzel, T., Versaw, W.K., Schwarzländer, M., and Lim, B.L., ATP compartmentation in plastids and cytosol of Arabidopsis thaliana revealed by fluorescent protein sensing, Proc. Natl. Acad. Sci. U.S.A., 2018, vol. 115, p. 10778. https://doi.org/10.1073/pnas.1711497115
Igamberdiev, A.U. and Kleczkowski, L.A., Implications of adenylate kinase-governed equilibrium of adenylates on contents of free magnesium in plant cells and compartments, Biochem. J., 2001, vol. 360, p. 225. https://doi.org/10.1042/0264-6021:3600225
Margalha, L., Confraria, A., and Baena-González, E., SnRK1 and TOR: modulating growth–defense trade-offs in plant stress responses, J. Exp. Bot., 2019, vol. 70, p. 2261. https://doi.org/10.1093/jxb/erz066
Baena-González, E., Rolland, F., Thevelein, J.M., and Sheen, J., A central integrator of transcription networks in plant stress and energy signaling, Nature, 2007, vol. 448, p. 938. https://doi.org/10.1038/nature06069
Nukarinen, E., Nägele, T., Pedrotti, L., Wurzinger, B., Mair, A., Landgraf, R., Börnke, F., Hanson, J., Teige, M., Baena-Gonzalez, E., Dröge-Laser, W., and Weckwerth, W., Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation, Sci. Rep., 2016, vol. 6, p. 31697. https://doi.org/10.1038/srep31697
Baena-González, E. and Hanson, J., Shaping plant development through the SnRK1–TOR metabolic regulators, Plant Biol., 2017, vol. 35, p. 152. https://doi.org/10.1016/j.pbi.2016.12.004
Crepin, N. and Rolland, F., SnRK1 activation, signaling, and networking for energy homeostasis, Plant Bio-l., 2019, vol. 51, p. 29. https://doi.org/10.1016/j.pbi.2019.03.006
Martínez-Barajas, E. and Coello, P., How do SnRK1 protein kinases truly work? Plant Sci., 2020, vol. 291, art. ID 110330. https://doi.org/10.1016/j.plantsci.2019.110330
Ruiz-Gayosso, A., Rodríguez-Sotres, R., Martínez-Barajas, E., and Coello, P., A role for the carbohydrate-binding module (CBM) in regulatory SnRK1 subunits: the effect of maltose on SnRK1 activity, Plant J., 2018, vol. 96, p. 163. https://doi.org/10.1111/tpj.14026
Rodrigues, A., Adamo, M., Crozet, P., Margalha, L., Confraria, A., Martinho, C., Elias, A., Rabissi, A., Lumbreras, V., González-Guzmán, M., Antoni, R., Rodriguez, P.L., and Baena-González, E., ABI1 and PP2CA phosphatases are negative regulators of Snf1-related protein kinase1 signaling in Arabidopsis, Plant Cell, 2013, vol. 25, p. 3871. https://doi.org/10.1105/tpc.113.114066
van Dam, T.-J., Zwartkruis, F.J., Bos, J.L., and Snel, B., Evolution of the TOR pathway, J. Mol. Evol., 2011, vol. 73, p. 209. https://doi.org/10.1007/s00239-011-9469-9
Morgutti, S., Negrini, N., Pucciariello, C., and Sacchi, G.A., Role of trehalose and regulation of its levels as a signal molecule to abiotic stresses in plants, in Plant Signaling Molecules: Role and Regulation Under Stressful Environments, Khan, M.I.R., Reddy, P. S., Ferrante, A., and Khan, N.A., Eds., Amsterdam: Elsevier, 2019, p. 581. https://doi.org/10.1016/C2017-0-03384-0
Qi, H., Xia, F.N., and Xiao, S., Autophagy in plants: physiological roles and post-translational regulation, J. Integr. Plant Biol., 2021, vol. 63, p. 161. https://doi.org/10.1111/jipb.12941
Shi, L., Wu, Y., and Sheen, J., TOR signaling in plants: conservation and innovation, Development, 2018, vol. 145, art. ID 160887. https://doi.org/10.1242/dev.160887
Dong, Y., Silbermann, M., Speiser, A., Forieri, I., Linster, E., Poschet, G., Samami, A.A., Wanatabe, M., Sticht, C., Teleman, A.A., Deragon, J.M., Saito, K., Hell, R., and Wirtz, M., Sulfur availability regulates plant growth via glucose-TOR signaling, Nat. Commun., 2017, vol. 8, p. 1174. https://doi.org/10.1038/s41467-017-01224-w
O’Leary, B.M., Oh, G.G.K., Lee, C.P., and Millar, A.H., Metabolite regulatory interactions control plant respiratory metabolism via target of rapamycin (TOR) kinase activation, Plant Cell, 2020, vol. 32, p. 666. https://doi.org/10.1105/tpc.19.00157
Janse van Rensburg, H.C., van den Ende, W., and Signorelli, S., Autophagy in plants: both a puppet and a puppet master of sugars, Front. Plant Sci., 2019, vol. 10, p. 14. https://doi.org/10.3389/fpls.2019.00014
Rabadanova, C.K., Tyutereva, E.V., Matskievich, V.S., Demidchik, V.V., and Voitsekhovskaya, O.V., Cellular and molecular mechanisms controlling autophagy: a perspective to improve plant stress resistance and crop productivity, S-kh. Biol., 2018, vol. 53, p. 881. https://doi.org/10.15389/agrobiology.2018.5.881eng
Izumi, M., Hidema, J., and Ishida, H., Deficiency of autophagy leads to significant changes of metabolic profiles in Arabidopsis, Plant Signaling Behav., 2013, vol. 8, p. e25023. https://doi.org/10.4161/psb.25023
van der Wilden, W., Herma, E.M., and Chrispeels, M.J., Protein bodies of mung bean cotyledons as autophagic organelles, Proc. Nat. Acad. Sci. U.S.A., 1980, vol. 77, p. 428. https://doi.org/10.1073/pnas.77.1.428
Toyooka, K., Okamoto, T., and Minamikawa, T., Cotyledon cells of Vigna mungo seedlings use at least two distinct autophagic machineries for degradation of starch granules and cellular components, J. Cell Biol., 2001, vol. 154, p. 973. https://doi.org/10.1083/jcb.200105096
Wang, Y., Zheng, X., Yu, B., Han, S., Guo, J., Tang, H., Yu, A.Y.L., Deng, H., Hong, Y., and Liu, Y., Disruption of microtubules in plants suppresses macroautophagy and triggers starch excess-associated chloroplast autophagy, Autophagy, 2015, vol. 11, p. 2259. https://doi.org/10.1080/15548627.2015.1113365
Marty, F., Plant vacuoles, Plant Cell, 1999, vol. 11, p. 587. https://doi.org/10.1105/tpc.11.4.587
Kazibwe, Z., Liu, A.Y., MacIntosh, G.C., and Bassham, D.C., The ins and outs of autophagic ribosome turnover, Cells, 2019, vol. 8, p. 1603. https://doi.org/10.3390/cells8121603
Davies, M., Singlet oxygen-mediated damage to proteins and its consequences, Biochem. Biophys. Res. Commun., 2003, vol. 305, p. 761. https://doi.org/10.1016/s0006-291x(03)00817-9
Barros, J.A.S., Cavalcanti, J.H.F., Medeiros, D.B., Nunes-Nesi, A., Avin-Wittenberg, T., Fernie, A.R., and Araújo, W.L., Autophagy deficiency compromises alternative pathways of respiration following energy deprivation in Arabidopsis thaliana, Plant Physiol., 2017, vol. 175, p. 62. https://doi.org/10.1104/pp.16.01576
Ambrosio, S. and Majello, B., Autophagy roles in genome maintenance, Cancers, 2020, vol. 12, p. 1793. https://doi.org/10.3390/cancers12071793
Nisa, M.U., Huang, Y., Benhamed, M., and Raynaud, C., The plant DNA damage response: signaling pathways leading to growth inhibition and putative role in response to stress conditions, Front. Plant Sci., 2019, vol. 10, p. 653. https://doi.org/10.3389/fpls.2019.00653
Fan, J., Yu, L., and Xu, C., Dual role for autophagy in lipid metabolism in Arabidopsis, Plant Cell, 2019, vol. 31, p. 1598. https://doi.org/10.1105/tpc.19.00170
Masclaux-Daubresse, C., d’Andrea, S., Bouchez, I., and Cacas, J.-L., Reserve lipids and plant autophagy, J. Exp. Bot., 2020, vol. 71, p. 2854. https://doi.org/10.1093/jxb/eraa082
Couso, I., Perez-Perez, M.E., Martinez-Force, E., Kim, H.-S., He, Y., Umen, J.G., and Crespo, J.L., Autophagic flux is required for the synthesis of triacylglycerols and ribosomal protein turnover in Chlamydomonas, J. Exp. Bot., 2018, vol. 69, p. 1355. https://doi.org/10.1093/jxb/erx372
Zhao, L., Dai, J., and Wu, Q., Autophagy-like processes are involved in lipid droplet degradation in Auxenochlorella protothecoides during the heterotrophy–autotrophy transition, Front. Plant Sci., 2014, vol. 5, p. 400. https://doi.org/10.3389/fpls.2014.00400
Sera, Y., Hanamata, S., Sakamoto, S., Ono, S., Kaneko, K., Mitsui, Y., Koyano, T., Fufita, N., Sasou, A., Masumura, T., Saji, H., Nonomura, K.I., Nitsuda, N., Mitsui, T., Kurusu, T., et al., Essential roles of autophagy in metabolic regulation in endosperm development during rice seed maturation, Sci. Rep., 2019, vol. 9, p. 18544. https://doi.org/:10.1038/s41598-019-54361-1
Herman, E.M. and Larkins, B.A., Protein storage bodies and vacuoles, Plant Cell, 1999, vol. 11, p. 601. https://doi.org/10.1105/tpc.11.4.601
Gifford, D.J. and Bewley, J.D., An analysis of the subunit structure of the crystalloid protein complex from castor bean endosperm, Plant Physiol., 1983, vol. 72, p. 376. https://doi.org/10.1104/pp.72.2.376
Goto-Yamada, S., Mano, S., Yamada, K., Oikawa, K., Hosokawa, Y., Hara-Nishimura, I., and Nishimura, M., Dynamics of the light-dependent transition of plant peroxisomes, Plant Cell Physiol., 2015, vol. 56, p. 1264. https://doi.org/10.1093/pcp/pcv081
Ren, K., Feng, L., Sun, S., and Zhuang, X., Plant mitophagy in comparison to mammals: what is still missing? Int. J. Mol. Sci., 2021, vol. 22, p. 1236. https://doi.org/10.3390/ijms22031236
Dmitrieva, V.A., Tyutereva, E.V., and Voitsekhovskaja, O.V., Singlet oxygen in plants: generation, detection, and signaling roles, Int. J. Mol. Sci., 2020, vol. 21, p. 3237. https://doi.org/10.3390/ijms21093237
Olmedilla, A. and Sandalio, L.M., Selective autophagy of peroxisomes in plants: from housekeeping to development and stress responses, Front. Plant Sci., 2019, vol. 10, p. 1021. https://doi.org/10.3389/fpls.2019.01021
Otegui, M.S., Vacuolar degradation of chloroplast components: autophagy and beyond, J. Exp. Bot., 2018, vol. 69, p. 741. https://doi.org/10.1093/jxb/erx234
Nakamura, S., Hagihara, S., Otomo, K., Ishida, H., Hidema, J., Nemoto, T., and Izumi, M., Autophagy contributes to the quality control of leaf mitochondria, Plant Cell Physiol., 2021, vol. 62, p. 229. https://doi.org/10.1093/pcp/pcaa162
Kikuchi, Y., Nakamura, S., Woodson, J.D., Ishida, H., Ling, Q., Hidema, J., Jarvis, R.P., Hagihara, S., and Izumi, M., Chloroplast autophagy and ubiquitination combine to manage oxidative damage and starvation responses, Plant Physiol., 2020, vol. 183, p. 1531. https://doi.org/10.1104/pp.20.00237
Woodson, J.D., Joens, M.S., Sinson, A.B., Gilkerson, J., Salomé, P.A., Weigel, D., Fitzpatrick, J.A., and Chory, J., Ubiquitin facilitates a quality-control pathway that removes damaged chloroplasts, Science, 2015, vol. 350, p. 450. https://doi.org/10.1126/science.aac7444
Xian, H. and Liou, Y.C., Functions of outer mitochondrial membrane proteins: mediating the crosstalk between mitochondrial dynamics and mitophagy, Cell Death Differ., 2021, vol. 28, p. 827. https://doi.org/10.1038/s41418-020-00657-z
Onishi, M., Yamano, K., Sato, M., Matsuda, N., and Okamoto, K., Molecular mechanisms and physiological functions of mitophagy, EMBO J., 2021, vol. 40, p. e104705. https://doi.org/10.15252/embj.2020104705
Ma, J., Liang, Z., Zhao, J., Wang, P., Ma, W., Andrade, J.A.F., Zeng, Y., Grujic, N., Jiang, L., Dagdas, Y., and Kang, B.H., Friendly mediates membrane depolarization-induced mitophagy in Arabidopsis, Curr. Biol., 2021, vol. 31, p. 1931. https://doi.org/10.1016/j.cub.2021.02.034
Minibayeva, F., Dmitrieva, S., Ponomareva, A., and Ryabovol, V., Oxidative stress-induced autophagy in plants: the role of mitochondria, Plant Physiol. Biochem., 2012, vol. 59, p. 11. https://doi.org/10.1016/j.plaphy.2012.02.013
Rose, R.J., Sustaining life: maintaining chloroplasts and mitochondria and their genomes in plants, Yale J. Biol. Med., 2019, vol. 923, p. 499.
Lorimer, G.H., Badger, M.R., and Andrews, T.J., The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications, Biochemistry, 1976, vol. 15, p. 529. https://doi.org/10.1021/bi00648a012
Sze, H. and Chanroj, S., Plant endomembrane dynamics: studies of K+/H+ antiporters provide insights on the effects of pH and ion homeostasis, Plant Physiol., 2018, vol. 177, p. 875. https://doi.org/10.1104/pp.18.00142
Gaxiola, R.A., Palmgren, M.G., and Schumacher, K., Plant proton pumps, FEBS Lett., 2007, vol. 581, p. 2204. https://doi.org/10.1016/j.febslet.2007.03.050
Trono, D., Laus, M.N., Soccio, M., Alfarano, M., and Pastore, D., Modulation of potassium channel activity in the balance of ROS and ATP production by durum wheat mitochondria–an amazing defense tool against hyperosmotic stress, Front. Plant Sci., 2015, vol. 6, p. 1072. https://doi.org/10.3389/fpls.2015.01072
Finazzi, G., Petroutsos, D., Tomizioli, M., Flori, S., Sautron, E., Villanova, V., Rolland, N., and Seigneurin-Berny, D., Ions channels/transporters and chloroplast regulation, Cell Calcium, 2015, vol. 58, p. 86. https://doi.org/10.1016/j.ceca.2014.10.002
Leigh, R.A. and Wyn Jones, R.G., A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell, New Phytol., 1984, vol. 97, p. 1.
Armengaud, P., Sulpice, R., Miller, T.J., Stitt, M., Amtmann, A., and Gibbon, Y., Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots, Plant Physiol., 2009, vol. 150, p. 772. https://doi.org/10.1104/pp.108.133629
Demidchik, V., Cuin, T.A., Svistunenko, D., Smith, S.J., Miller, A.J., Shabala, S., Sokolik, A., and Yurin, V., Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death, J. Cell Sci., 2010, vol. 123, p. 1468. https://doi.org/10.1104/pp.108.133629
Demidchik, V., Tyutereva, E.V., and Voitsekhovskaja, O.V., The role of ion disequilibrium in induction of root cell death and autophagy by environmental stresses, Funct. Plant Biol., 2018, vol. 45 P. 28. https://doi.org/10.1071/FP16380
Floyd, B.E., Pu, Y., Soto-Burgos, J., and Bassham, D., To live or die: autophagy in plants, in Plant Programmed Cell Death, Cham: Springer-Verlag, 2015, p. 269. https://doi.org/10.1007/978-3-319-21033-9_11
Üstün, S., Hafrén, A, and Hofius, D., Autophagy as a mediator of life and death in plants, Curr. Opin. Plant Biol., 2017, vol. 40, p. 122. https://doi.org/10.1016/j.pbi.2017.08.011
Zhou, L.L., Gao, K.Y., Cheng, L.S., Wang, Y.L., Cheng, Y.K., Xu, Q.T., Deng, X.Y., Li, J.W., Mei, F.Z., and Zhou, Z.Q., Short-term waterlogging-induced autophagy in root cells of wheat can inhibit programmed cell death, Protoplasma, 2021, vol. 258, p. 891. https://doi.org/10.1007/s00709-021-01610-8
van Doorn, W.G., Beers, E.P., Dangl, J.L., Franklin-Tong, V.E., Gallois, P., Hara-Nishimura, I., Jones, A.M., Kawai-Yamada, M., Lam, E., Mundy, J., Mur, L.A.J., Petersen, M., Smertenko, A., Taliansky, M., van Breusegem, F., et al., Morphological classification of plant cell deaths, Cell Death Differ., 2011, vol. 18, p. 1241. https://doi.org/10.1038/cdd.2011.36
Valandro, F., Menguer, P.K., Cabreira-Cagliari, C., Margis-Pinheiro, M., and Cagliari, A., Programmed cell death (PCD) control in plants: new insights from the Arabidopsis thaliana deathosome, Plant Sci., 2020, vol. 299, art. ID 110603. https://doi.org/10.1016/j.plantsci.2020.110603
Hofius, D., Munch, D., Bressendorff, S., Mundy, J., and Petersen, M., Role of autophagy in disease resistance and hypersensitive response-associated cell death, Cell Death Differ., 2011, vol. 18, p. 1257. https://doi.org/10.1038/cdd.2011.43
Hackenberg, T., Juul, T., Auzina, A., Gwiżdż, S., Małolepszy, A., van der Kelen, K., Dam, S., Bressendorff, S., Lorentzen, A., Roepstorff, P., Nielsen, K.L., Jørgensen, J.-E., Hofius, D., van Breusegem, F., Petersen, M., et al., Catalase and NO CATALASE ACTIV-ITY1 promote autophagy-dependent cell death in Arabidopsis, Plant Cell, 2013, vol. 5, p. 4616. https://doi.org/10.1105/tpc.113.117192
Tyutereva, E.V., Dobryakova, K.S., Schiermeyer, A., Shishova, M.F., Pawlowski, K., Demidchik, V., Reumann, S., and Voitsekhovskaja, O.V., The levels of peroxisomal catalase protein and activity modulate the onset of cell death in tobacco BY-2 cells via reactive oxygen species levels and autophagy, Funct. Plant Biol., 2018, vol. 45, p. 247. https://doi.org/10.1071/FP16418
Teper-Bamnolker, P., Danieli, R., Peled-Zehavi, H., Belausov, E., Abu-Abied, M., Avin-Wittenberg, T., Sadot, E., and Eshel, D., Vacuolar processing enzyme translocates to the vacuole through the autophagy pathway to induce programmed cell death, Autophagy, 2020, vol. 19, p. 1. https://doi.org/10.1080/15548627.2020.1856492
Voitsekhovskaja, O.V., Schiermeyer, A., and Reumann, S., Plant peroxisomes are degraded by starvation-induced and constitutive autophagy in tobacco BY-2 suspension-cultured cells, Front. Plant Sci., 2014, vol. 5, p. 629. https://doi.org/10.3389/fpls.2014.00629
ACKNOWLEDGMENTS
We thank Dr. Katharina Pawlowski (Stockholm University, Stockholm, Sweden) for critical reading of the English version of this manuscript.
Funding
This review was funded by RFBR, project number 20-14-50480. In the review, we used results of the projects supported by the Russian Science Foundation (nos. 18-16-00074 and 14-16-00120-P). We apologize to the authors whose works could not be cited due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Additional information
This article is dedicated to the memory of Olga Aleksandrovna Semikhatova who studied the role of respiration in the energy balance in plants, and dedicated to her 100th anniversary.
The article was translated by the authors.
Abbreviations: АК—adenylate kinase; PCD—programmed cell death; TAG—triacylglycerol; ER—the endoplasmic reticulum; AIM—ATG8-interacting motif; AKIN—Arabidopsis KIN; AMPK—AMP-dependent kinase; ATI—ATG8-interacting protein 1; ATG—AuTophaGy-related genes; bZIP—Basic Leucine Zipper; CBM—carbohydrate binding module; CHMP1— Charged Multivesicular Body 1; DCMU—3-(3,4-dichlorophenyl)-1,1-dimethylurea; eATP—extracellular ATP; FMT— friendly mitochondria; GRIK1,2—geminivirus Rep interacting kinase1,2; GORK—guard cell outward-rectifying K+ channel; GTP—guanosine triphosphate; KIN—catalytic subunit of the SnRK1 protein kinase; LST8—lethal with SEC-thirteen protein 8; MgADP—magnesium-chelated ADP; MgATP—magnesium-chelated ATP; PAS—phagophore assembly site; PM-ANT1—Plasma Membrane Adenine nucleotide translocator 1; PP2Cs—Protein Phosphatase 2c; PUB4—plant U-box 4; RAPTOR—Regulatory-associated protein of TOR; RCBs—Rubisco-containing bodies; RGS—Regulator of G-protein signaling gene; SAPK—stress-activated MAP kinase; SIN1—SAPK-interacting protein 1; SnAK—SnRK-Activating Kinase; SNF1—Sucrose non-fermenting 1; SnRK1—Snf1-related protein kinase 1; SSGL—small starch granule-like structure; Т6Р—trehalose-6-phosphate; TOR—target of rapamycin; UIM—ubiquitin-interacting motif; VPE—vacuolar processing enzyme.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tyutereva, E.V., Murtuzova, A.V. & Voitsekhovskaja, O.V. Autophagy and the Energy Status of Plant Cells. Russ J Plant Physiol 69, 19 (2022). https://doi.org/10.1134/S1021443722020212
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722020212