Abstract
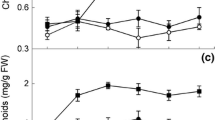
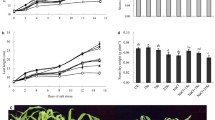
This study was performed to understand the mechanisms for Se-enhanced resistance of parsley (Petroselinum crispum L.) plants to salinity stress. Plant growth was negatively affected by salt stress; however, Se treatments at 1 mg/L significantly improved the growth rate and enhanced the salt tolerance of seedlings. This increased tolerance in Se-supplied plants was obtained by reduced damaging effect on maximal quantum yield of photosystem II (PSII) (F v/F m) coupled with higher levels of carotenoids and non-photochemical quenching (NPQ). The performance index (PIABS), as evidence for modulation of PSII function, was downregulated by salt stress; while Se mitigated this effect. Moreover, analysis of OJIP transients demon-strated that Se reduced salt damaging effect on PSII function through improvement of excitation energy trapping (TR0/CS) and electron transport (ET0/CS) per excited cross-section of leaf. The Na concentrations in shoots and roots of parsley seedlings considerably enhanced after NaCl treatment. Interestingly, treatment of salt-stressed plants with Se decreased the Na contents in shoots via the limitation of the root-to-shoot translocation of Na and exclusion of Na from cell sap, as well as the retention of K/Na and Ca/Na ratios. These data provide the first evidence that the Se application alleviates salinity stress by enhancing PSII function and by decreasing Na content in the shoot via binding of Na to the root cell wall.
Similar content being viewed by others
Abbreviations
- ABS/RC:
-
specific energy fluxes for absorption
- DI0/RC:
-
dissipation at the level of the antenna chlorophylls
- Chl:
-
chlorophyll
- ET0/RC:
-
dissipation at the level of electron transport
- F v/F m :
-
maximum quantum yield of PSII
- NPQ :
-
non-photochemical quenching
- PIABS :
-
performance indexes
- TR0/RC:
-
specific energy fluxes for trapping
References
Munns, R. and Tester, M., Mechanisms of salinity tolerance, Annu. Rev. Plant Biol., 2008, vol. 59, pp. 651–681.
Diao, M., Ma, L., Wang, J.W., Cui, J.X., Fu, A.F., and Liu, H.Y., Selenium promotes the growth and photo-synthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system, J. Plant Growth Regul., 2014, vol. 33, pp. 671–682.
Demidchik, V., Cuin, T.A., Svistunenko, D., Smith, S.J., Miller, A.J., Shabala, S., Sokolik, A., and Yurin, V., Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death, J. Cell Sci., 2010, vol. 123, pp. 1468–1479.
Hasanuzzaman, M., Hossain, M.A., and Fujita, M., Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings, Biol. Trace Elem. Res., 2011, vol. 143, pp. 1704–1721.
Kong, L., Wang, M., and Bi, D., Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress, Plant Growth Regul., 2005, vol. 45, pp. 155–163.
Hawrylak-Nowak, B., Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress, Biol. Trace Elem. Res., 2009, vol. 132, pp. 259–269.
Maxwell, K. and Johnson, G.N., Chlorophyll fluorescence— a practical guide, J. Exp. Bot., 2000, vol. 51, pp. 659–668.
Van Heerden, P.D.R., Swanepoel, J.W., and Krüger, G.H.J., Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation, Environ. Exp. Bot., 2007, vol. 61, pp. 124–136.
Brestič, M. and Živčák, M., PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: protocols and applications, in Molecular Stress Physiology of Plants, Rout, G.R. and Das, A.B., Eds., Berlin, Heidelberg: Springer-Verlag, 2013, pp. 87–131.
Strasser, R.J., Tsimilli-Michael, M., and Srivastava, A., Analysis of the chlorophyll a fluorescence transient, in Advances in Photosynthesis and Respiration, vol. 19, Chlorophyll a Fluorescence: A Signature of Photosynthesis, Papageorgiou, G.C. and Govindjee, Eds., Dordrecht: Springer-Verlag, 2004, pp. 321–362.
Álvaro, J.E., Lao, M.T., Urrestarazu, M., Baghour, M., and Abdelmajid, M., Effect of nutrient solution salinity and ionic concentration on parsley (Petroselinum crispum Mill.) essential oil yield and content, J. Plant Nutr., 2015, vol. 37, pp. 2236–2254.
Mazej, D., Osvald, J., and Stibilj, V., Selenium species in leaves of chicory, dandelion, lamb’s 351 lettuce and parsley, Food Chem., 2007, vol. 107, pp. 75–83.
Johnson, C.M., Stout, P.R., Broyer, T.C., and Carlton, A.B., Comparative chlorine requirements of different plant species, Plant Soil, 1957, vol. 8, pp. 337–353.
Lichtenthaler, H.K. and Wellburn, A.R., Determination of total carotenoids and chlorophylls a and b of leaf in different solvents, Biochem. Soc. Trans., 1985, vol. 11, pp. 591–592.
Saqib, M., Zörb, C., and Schubert, S., Silicon-mediated improvement in the salt resistance of wheat (Triticum aestivum) results from increased sodium exclusion and resistance to oxidative stress, Funct. Plant Biol., 2008, vol. 35, pp. 633–639.
Rogalla, H. and Römheld, V., Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L., Plant Cell Environ., 2002, vol. 25, pp. 549–555.
Živčák, M., Olšovská, K., Slamka, P., Galambošová, J., Rataj, V., Shao, H.B., and Brestič, M., Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency, Plant Soil Environ., 2014, vol. 60, pp. 210–215.
Lazár, D., The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light, Funct. Plant Biol., 2006, vol. 33, pp. 9–30.
Baker, N.R. and Rosenqvist, E., Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities, J. Exp. Bot., 2004, vol. 55, pp. 1607–1621.
Vaz, J. and Sharma, P.K., Relationship between xanthophyll cycle and non-photochemical quenching in rice (Oryza sativa L.) plants in response to light stress, Indian J. Exp. Bot., 2011, vol. 49, pp. 60–67.
Davey, M.W., Stals, E., Panis, B., Keulemans, J., and Swennen, R.L., High-through put determination of malondialdehyde in plant tissues, Anal. Biochem., 2005, vol. 347, pp. 201–207.
Azzabi, G., Pinnola, A., Betterle, N., Bassi, R., and Alboresi, A., Enhancement of non-photochemical quenching in the bryophyte Physcomitrella patens during acclimation to salt and osmotic stress, Plant Cell Physiol., 2012, vol. 53, pp. 1815–1825.
Takahashi, S. and Badger, M.R., Photoprotection in plants: a new light on photosystem II damage, Trends Plant Sci., 2011, vol. 16, pp. 53–60.
Murchie, E.H. and Niyogi, K.K., Manipulation of photoprotection to improve plant photosynthesis, Plant Physiol., 2011, vol. 155, pp. 86–92.
Hajiboland, R. and Keivanfar, N., Selenium supplementation stimulates vegetative and reproductive growth in canola (Brassica napus L.) plants, Acta Agric. Slov., 2012, vol. 99, pp. 13–19.
Cazzonelli, C.I. and Pogson, B.J., Source to sink: regulation of carotenoid biosynthesis in plants, Trends Plant Sci., 2010, vol. 15, pp. 266–274.
Habibi, G. and Ajory, N., The effect of drought on photosynthetic plasticity in Marrubium vulgare plants growing at low and high altitudes, Plant Res., 2015, vol. 128, pp. 987–994.
Zheng, Y., Jia, A., Ning, T., Xu, J., Li, Z., and Jiang, G., Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance, J. Plant Physiol., 2008, vol. 165, pp. 1455–1465.
Hajiboland, R., Norouzi, F., and Poschenrieder, C., Growth, physiological, biochemical and ionic responses of pistachio seedlings to mild and high salinity, Trees, 2014, vol. 28, pp. 1065–1078.
Pazurkiewicz-Kocot, K., Galas, W., and Kita, A., The effect of selenium on the accumulation of some metals in Zea mays L. plants treated with indole-acetic acid, Cell Mol. Biol. Lett., 2003, vol. 8, pp. 97–103.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Habibi, G. Selenium ameliorates salinity stress in Petroselinum crispum by modulation of photosynthesis and by reducing shoot Na accumulation. Russ J Plant Physiol 64, 368–374 (2017). https://doi.org/10.1134/S1021443717030086
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443717030086