Abstract
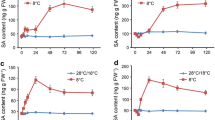
Proline metabolism is implicated in plant responses to abiotic stresses, including the chilling stress. During proline catabolism, the two-step oxidation of proline is performed by the continuous actions of proline dehydrogenase (ProDH), which produces Δ1-pyrroline-5-carboxylate (P5C), and P5C dehydrogenase (P5CDH), which oxidizes P5C to glutamate. The Arabidopsis thaliana chilling mutants chs1 and chs2 are sensitive to chilling temperatures of 13–18°C. For a better understanding of Arabidopsis responses to chilling stress, 4-week-old wild-type (WT) and chs1 and chs2 lines, with three plants in each group, were subjected to chilling stress (13°C), cold stress (4°C), or remained under normal conditions (23°C); and several factors including the expression of ProDH2 and P5CDH genes, POX (peroxidase) and SOD (superoxide dismutase) activities, as well as MDA and proline contents were examined. Our results showed an increase in the proline content in all lines under chilling conditions. In addition, a greater expression of ProDH2 and a lower expression of P5CDH were observed, leading us to speculate a greater breakdown of proline into P5C and a consequent overproduction of ROS in the ETC cycle. The higher POX and SOD activities and a higher MDA content in chs mutants at 13°C are in line with this speculation. Finally, cold-treated plants (4°C) only showed an increase in proline levels.
Similar content being viewed by others
Abbreviations
- chs :
-
chilling-sensitive
- ETC:
-
electron transport chain
- GR:
-
glutathione reductase
- P5C:
-
Δ1-pyrroline-5-carboxylate
- PCD:
-
programmed cell death
- P5CDH:
-
P5C dehydrogenase
- P5CR:
-
P5C reductase
- P5CS:
-
P5C synthetase
- POX:
-
peroxidase
- ProDH:
-
proline dehydrogenase
- SOD:
-
superoxide dismutase
- WT:
-
wild-type
References
Zhou, B.Y., Guo, Z.F., and Liu, Z.L., Effects of Abscisic Acid on Antioxidant Systems of Stylosanthes guianensis (Aublet) Sw. under Chilling Stress, Crop Sci., 2005, vol. 45, pp. 599–605.
Lyons, J.M., Chilling Injury in Plants, Annu. Rev. Plant Physiol., 1973, vol. 24, pp. 445–466.
Schneider, J.C., Hugly, S., and Somerville, C.R., Chilling Sensitive Mutants of Arabidopsis, Weeds World, 1994, vol. 1, pp. 11–17.
Schneider, J.C., Nielsen, E., and Somerville, C.R., A Chilling-Sensitive Mutant of Arabidopsis Is Deficient in Chloroplast Protein Accumulation at Low Temperature, Plant Cell Environ., 1995, vol. 18, pp. 23–32.
Provart, N.J., Gil, P., Chen, W., Han, B., Chang, H.-S., Wang, X., and Zhu, T., Gene Expression Phenotypes of Arabidopsis Associated with Sensitivity to Low Temperatures, Plant Physiol., 2003, vol. 132, pp. 893–906.
Zoldan, D., Shekaste, Band, R., Guy, C.L., and Porat, R., Understanding Chilling Tolerance Traits Using Arabidopsis Chilling-Sensitive Mutants, Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change, Ahmad, P. and Prasad, M.N.V., Eds., eBook, Springer-Verlag, 2012, pp. 159–173.
Hugly, S., McCourt, P., Browse, J., Patterson, G.W., and Somersville, C., A Chilling Sensitive Mutant of Arabidopsis with Altered Steryl-Ester Metabolism, Plant Physiol., 1990, vol. 93, pp. 1053–1062.
Patterson, G.W., Hugly, S., and Harrison, D., Sterols and Phytyl Esters of Arabidopsis thaliana under Normal and Chilling Temperatures, Phytochemistry, 1993, vol. 33, pp. 1381–1383.
Yamaguchi-Shinozaki, K. and Shinozaki, K., Organization of cis-Acting Regulatory Elements in Osmotic- and Cold-Stress-Responsive Promoters, Trends Plant Sci., 2005, vol. 10, pp. 88–94.
Delauney, A.J. and Verma, D.P.S., Proline Biosynthesis and Osmoregulation in Plants, Plant J., 1993, vol. 4, pp. 215–223.
Elthon, T.E. and Stewart, C.R., Submitochondrial Location and Electron Transport Characteristics of Enzymes Involved in Proline Oxidation, Plant Physiol., 1981, vol. 67, pp. 780–784.
Cecchini, N.M., Monteoliva, M.I., and Alvarez, M.E., Proline Dehydrogenase Is a Positive Regulator of Cell Death in Different Kingdoms, Plant Signal. Behav., 2011, vol. 6, pp. 1195–1197.
Shinozaki, K. and Yamaguchi-Shinozaki, K., Molecular Responses to Dehydration and Low Temperature: Differences and Cross-Talk between Two Stress Signaling Pathways, Curr. Opin. Plant Biol., 2000, vol. 3, pp. 217–223.
Heath, R.L. and Packer, L., Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation, Arch. Biochem. Biophys., 1968, vol. 125, pp. 189–198.
Bradford, M.M., A Rapid and Sensitive Method for the Quantization of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Abeles, F.B. and Biles, C.L., Characterization of Peroxidase in Lignifying Peach Fruit Endocarp, Plant Physiol., 1991, vol. 95, pp. 269–273.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.L., Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem., 1951, vol. 193, pp. 265–275.
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid Determination of Free Proline for Water-Stress Studies, Plant Soil, 1973, vol. 39, pp. 205–207.
Dhindsa, R.S., Plumb-Dhindsa, P., and Thorpe, T.A., Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase, J. Exp. Bot., 1981, vol. 32, pp. 93–101.
Xu, S.C., Li, Y.-P., Hu, J., Guan, Y.J., Ma, W.G., Zheng, Y.Y., and Zhu, S.J., Responses of Antioxidant Enzymes to Chilling Stress in Tobacco Seedlings, Agric. Sci. China, 2010, vol. 9, pp. 1594–1601.
Li, Q., Yu, B., Gao, Y., Dai, A.H., and Bai, J.G., Cinnamic Acid Pretreatment Mitigates Chilling Stress of Cucumber Leaves through Altering Antioxidant Enzyme Activity, J. Plant Physiol., 2011, vol. 168, pp. 927–934.
Lee, D.F. and Lee, C.B., Chilling Stress-Induced Changes of Antioxidant Enzymes in the Leaves of Cucumber: In Gel Enzyme Activity Assays, Plant Sci., 2000, vol. 159, pp. 75–85.
O’Kane, D., Gill, V., Boyd, P., and Burdon, R., Chilling Oxidative Stress and Antioxidant Responses in Arabidopsis thaliana Callus, Planta, 1996, vol. 198, pp. 371–377.
Peng, Z., Lu, Q., and Verma, D.P., Reciprocal Regulation of Delta 1-Pyrroline-5-Carboxylate Synthetase and Proline Dehydrogenase Genes Controls Proline Levels during and after Osmotic Stress in Plants, Mol. Gen. Genom., 1996, vol. 253, pp. 334–341.
Phang, J.M., Pandhare, J., Zabirnyk, O., and Liu, Y., The Metabolism of Proline as Microenvironmental Stress Substrate, J. Nutr., 2008, vol. 138, pp. 2008–2015.
Deuschle, K., Funck, D., Forlani, G., Stransky, H., Biehl, A., Leister, D., van der Graaff, E., Kunze, R., and Frommer, W.B., The Role of Δ1-Pyrroline-5-Carboxylate Dehydrogenase in Proline Degradation, Plant Cell, 2004, vol. 16, pp. 3413–3425.
Szekely, G., Abraham, E., Cseplo, A., Rigo, G., Zsigmond, L., Csiszar, J., Ayaydin, F., Strizhov, N., Jasik, J., Schmeizer, E., Koncz, C., and Szabados, L., Duplicated P5CS Genes of Arabidopsis Play Distinct Roles in Stress Regulation and Developmental Control of Proline Biosynthesis, Plant J., 2008, vol. 53, pp. 11–28.
Miller, M.R., Borthwick, S.J., Shaw, C.A., McLean, S.G., McClure, D., Mills, N.L., Duffin, R., Donaldson, K., Megson, I.L., Hadoke, P.W.F., and Newby, D.E., Direct Impairment of Vascular Function by Diesel Exhaust Particulate through Reduced Bioavailability of Endothelium-Derived Nitric Oxide Induced by Superoxide Free Radicals, Environ. Health Perspect., 2009, vol. 117, pp. 611–616.
Author information
Authors and Affiliations
Corresponding author
Additional information
This text was submitted by the authors in English.
This text was submitted in English.
Rights and permissions
About this article
Cite this article
Khavari-Nejad, R.A., Shekaste Band, R., Najafi, F. et al. The role of Pro-P5C Cycle in chs mutants of Arabidopsis under cold stress. Russ J Plant Physiol 60, 375–382 (2013). https://doi.org/10.1134/S1021443713020106
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443713020106