Abstract
Amino-containing porous aromatic frameworks, designated as PAF-20–NH2-pre/post and PAF-30–NH2-pre/post, were synthesized using a combination of pre- and post-modification, and were then used to prepare a number of Pd catalysts with various metal concentrations. The activity of the catalysts was tested in selective hydrogenation of a series of alkynes and dienes at 60°C and 10 atm hydrogen pressure. The effects of the modification technique and palladium content on the morphology and catalytic activity of nanoparticles were investigated, and the reaction patterns were identified for each substrate type. The reusability of the catalysts over at least six reaction cycles was demonstrated.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent years, porous organic polymers have attracted increasing attention among researchers due to the ability to tune finely their structural properties to match various process designs. An illustrative example of these materials are porous aromatic frameworks (PAFs)—polymers consisting of covalently bonded benzene rings [1, 2]. This class of organic polymers is distinguished by a three-dimensional porous structure (and, hence, a high specific surface area), high thermal and mechanical stability, and the possibility to modify the support’s structure by well-studied and relatively simple methods [3, 4]. All these advantages have made porous aromatic frameworks promising materials for such applications as heterogeneous catalysis, separation and storage of gases, and adsorption of heavy metals.
One of the key PAF features is their ability to stabilize transition metal nanoparticles, an active phase type that has been considered very promising for organic catalysis and petroleum chemistry [5–9]. The extraordinarily high catalytic activity of these nanoparticles is attributed to their high surface to volume ratio. At the same time, however, this high ratio is responsible for rapid aggregation and, therefore, deactivation of the nanoparticles. The use of PAFs as supports overcomes this challenge and facilitates the catalyst removal from the reaction mixture [10, 11]. In addition, modification of PAFs with various functional groups enables the control of morphology and electronic properties of nanoparticles as well as the geometry of their active sites, thus affecting the activity and selectivity of the catalysts [12, 13].
There are two strategies for modifying a PAF structure: pre- and post-functionalization [14–16]. The first approach is based on the use of modified monomers in the synthesis of supports to establish a specific arrangement of functional groups in the material. Unfortunately, the synthesis of such monomers is often overly time-consuming. Post-functionalization involves modification of a framework that has already been synthesized. However, while easier to implement, this method results in a random arrangement of functional groups. It is a combination of the two approaches that allows the concentration and position of the functional groups in the support to be adjusted, thus favoring the accessibility, stability, and catalytic properties of nanoparticles.
Proper control of the morphology and environment of transition metal nanoparticles is of particular importance in selective hydrogenation of dienes and acetylenes [17–19]. This process has been extensively implemented both in fine organic synthesis and in the industrial manufacture of olefin-derived products such as plastics, lubricants, and detergents [20, 21]. The most popular catalysts for this reaction have been a Lindlar catalyst (lead-modified palladium supported on CaCO3) [22, 23] and Pd-NanoSelect™ (supported colloidal palladium) [24, 25]. Unfortunately, both catalytic systems have a number of major drawbacks: lead-induced toxicity, non-tunable morphology of the active phase, and high quinoline consumption for Lindlar catalysts; and limited stability and uneven distribution of nanoparticles on the support surface in the case of Pd-NanoSelect™. Thus, the development of supports that would allow effective immobilization of transition metal nanoparticles and control of their morphology, catalytic activity, and stability without the need for additives or toxic promoters remains an urgent problem.
The purpose of this study was to investigate the dependence of the morphology and activity of palladium catalysts in the hydrogenation of alkynes and dienes with different chain lengths on the structure of amine-containing porous aromatic frameworks synthesized by a combination of post- and pre-modification strategies.
EXPERIMENTAL
Initial Materials
The following reagents were used to synthesize catalysts: palladium (II) acetate, Pd(OAc)2 (Sigma-Aldrich, 99.9+%); sodium borohydride, NaBH4 (Aldrich, 98%); and ethanol (IRea 2000, Russia, CP grade).
Phenylacetylene (ABCR, 98%), 1-hexyne (Aldrich, 98%), 1-octyne (ABCR, 98%), 4-octyne (ABCR, 98%), 2,5-dimethylhexadiene-2,4 (Aldrich, 98%), and isoprene (Aldrich, 98%) were used as substrates.
The following reagents were used to synthesize PAF samples, namely PAF-20–NH2-pre/post and PAF-30–NH2-pre/post: 1,4-phenylenediboric acid (ABCR, 96%); 4,4′-biphenyldiboric acid (ABCR, 97%); triphenylphosphine, PPh3 (Aldrich, ReagentPlus®, 99%); palladium (II) acetate, Pd(OAc)2 (Sigma-Aldrich, 99.9%); nitric acid, HNO3 (Component-Reaktiv, Russia, CP grade); sulfuric acid, H2SO4 (Component-Reaktiv, Russia, CP grade); and tin(II) chloride, SnCl2·2H2O (Aldrich, 98%). DMF (Rushim, Russia, high purity), THF (Component-Reaktiv, Russia, high purity), and trifluoroacetic acid (Aldrich, ReagentPlus®) were used as solvents. A tetrakis(4-bromo-3-nitrophenyl)methane monomer was synthesized by the method described in [3]. The characterizations of the materials are provided in the “Results and Discussion” section below.
Instruments and Methods
Low-temperature nitrogen adsorption/desorption. The porosity properties of the samples were measured on a Micromeritics Gemini VII 2390 (V1.02t) analyzer by a standard technique. Prior to testing, the samples were evacuated at 120°C and 3×10–3 atm for 6 h. The nitrogen adsorption/desorption isotherms were recorded at 77 K. The porosity of the structures was calculated using standard software. The specific surface area was derived from the adsorption data in the relative partial pressure (P/P0) range of 0.05–0.2 using a BET model. The total pore volume was calculated using a BJH model at P/P0 = 0.95.
IR spectroscopy. The samples were analyzed by Fourier-transform infrared (FT-IR) spectroscopy using a Thermo Scientific Nicolet IR200 instrument with a Multireflection HATR attenuation total reflection attachment (with a 45° ZnSe crystal for various wavelength ranges at a resolution of 4 nm in the range of 4000–400 cm–1). The spectra were obtained by averaging 100 scans.
Elemental analysis. The metal concentration in the catalysts was measured by inductively coupled plasma atomic emission spectrometry (ICP-AES) on a SHIMADZU ICPE-9000 spectrometer. The nitrogen content in the materials was examined using equipment provided by the Molecular Structure Research Center of the Institute of Organoelement Compounds of Russian Academy of Sciences (INEOS RAS).
TEM. The catalysts were examined by transmission electron microscopy (TEM) on a Libra 200 FE HR instrument with an accelerating voltage of 200 kV.
GC. The reaction products were analyzed by gas-liquid chromatography (GC) on a Hewlett-Packard chromatograph equipped with a flame ionization detector (FID) and a 50 m×0.32 mm×0.52 μm column (HP-1 grafted phase). Helium at a constant pressure of 1.5 atm was used as a carrier gas. The chromatograms were recorded and analyzed on a computer using the HP ChemStation Rev. A. 06. 01 (403) package.
Synthesis of PAFs
Synthesis of PAF-20–NO2-pre. Into a 100 mL one-neck flask equipped with a magnetic stir bar, tetrakis(4-bromo-3-nitrophenyl)methane (500 mg, 0.6 mmol) and 1,4-phenylenediboric acid (203 mg, 1.2 mmol) were dissolved in 25 mL of DMF. Palladium acetate (14 mg, 0.06 mmol), triphenylphosphine (88 mg, 0.33 mmol), and a 2 M potassium carbonate solution (3.5 mL, 7 mmol) were then added. The resultant solution was degassed by the freeze–pump–thaw technique, after which the flask was equipped with a reflux condenser, and the reaction was carried out under vigorous stirring at 140°C for 24 h. The precipitate was filtered and washed with 50 mL of water, then transferred into a 100 mL cup and treated for 40 minutes with a concentrated HCl (40 mL) solution in water (40 mL) with H2O2 (500 μL) being added to remove Pd residues. Next, the precipitate was filtered again and washed with water (2×50 mL), ethanol (2×50 mL), methylene chloride (50 mL), and THF (2×50 mL). After drying in vacuo for 6 h, PAF-20–NO2-pre was obtained as a beige powder (467 mg).
Synthesis of PAF-30–NO2-pre. A procedure similar to the one described above was followed. Tetrakis(4-bromo-3-nitrophenyl)methane (500 mg, 0.6 mmol) and 4,4′-biphenyldiboric acid (298 g, 1.2 mmol) were used as initial materials. The product was obtained as a light yellow powder (494 mg).
Synthesis of PAF-20–NO2-pre/post. Into a 50 mL three-necked flask equipped with a magnetic stir bar and a reflux condenser and held in an ice bath, 20 mL of pre-cooled to 0°C trifluoroacetic acid was added followed by 406 mg of PAF-20–NO2-pre. After stirring the resultant suspension for 10 min, 142 μL of nitric acid was slowly added. The suspension was then stirred at room temperature for 24 h and poured into ice. The precipitate was filtered and washed with water (2×50 mL) and ethanol (2×50 mL). The product was a yellow powder (387 mg).
Synthesis of PAF-30–NO2-pre/post. A procedure similar to that described above for PAF-20–NO2-pre/post was followed. PAF-30–NO2-pre (450 mg) was used as an initial material. The product was obtained as a light yellow powder (448 mg).
Synthesis of PAF-20–NH2-pre/post. In a 250 mL one-neck flask equipped with a magnetic stir bar, 380 mg of PAF-20–NO2-pre/post and 100 mL of THF were placed, followed by the addition of tin chloride (7 g, 31 mmol). The mixture was boiled under stirring for 24 h, followed by cooling, adding 50 mL of a 10% NaOH solution, and separating a solid product by filtering. The resultant precipitate was stirred repeatedly in a 10% NaOH solution to completely remove residual tin compounds, after which the precipitate was washed with water (2×50 mL) and THF (2×50 mL). The product was a brown powder (369 mg).
Synthesis of PAF-30–NH2-pre/post. A procedure similar to that described above for PAF-20–NH2-pre/post was followed. PAF-30–NO2-pre/post (400 mg) was used as an initial material. The product was obtained as a brown powder (398 mg).
Synthesis of Catalysts
In a 25 mL one-neck flask equipped with a magnetic stir bar and a reflux condenser, Pd(OAc)2 solution (4.3 mg, 0.019 mmol in the case of 2% Pd; 11.1 mg, 0.049 mmol for 5%Pd) was prepared in 10 mL of methylene chloride. Then 100 mg of a needed PAF was added to the solution, and the mixture was stirred for 24 h. Next, the mixture was vaporized using a rotary evaporator, and 8 mL of ethanol was added to the dry residue. After this, under vigorous stirring, 10 mL of a cooled NaBH4 solution (20 mg, 0.53 mmol) in a water/methanol system (1 : 1 v/v) was added dropwise to the suspension, followed by stirring for another 24 h. The resultant gray precipitate was filtered and washed with water (2×50 mL) and ethanol (2×50 mL), followed by vacuum-drying for 6 h.
Catalytic Tests
Hydrogenation was carried out in a steel autoclave equipped with an insert tube and a magnetic stir bar. Specific amounts of reactants (1 mg of a catalyst and a substrate volume derived from a substrate to metal molar ratio of 20 000) were placed into the tube, after which the autoclave was sealed, filled with hydrogen to 10 atm, and connected to a thermostat at 60°C. At the end of the reaction, the autoclave was cooled below room temperature and depressurized. The reaction products were analyzed by gas chromatography.
RESULTS AND DISCUSSION
Characterization of Catalysts
The amino-modified PAFs were synthesized by the combination of pre- and post-modification (Fig. 1). Initially, a tetrakis(4-bromo-3-nitrophenyl)methane monomer was prepared by treating tetrakis(4-bromophenyl)methane with a nitric/sulfuric acid mixture. This monomer was then used in a Suzuki–Miyaura cross-coupling with 1,4-phenylenediboric acid or 4,4′-biphenyldiboric acid, to synthesize PAF-20–NO2-pre or PAF-30–NO2-pre, respectively. The monomer determined the location of the nitro groups in the frameworks, and the linkers differing in the number of aromatic rings made it possible to vary the pore sizes. Thereafter, the materials were treated with nitric acid to introduce additional nitro groups into the PAF, thus preparing PAF-20–NO2-pre/post and PAF-30–NO2-pre/post. Finally, these samples were reduced with tin (II) chloride to synthesize the amino-modified PAF-20–NH2-pre/post and PAF-30–NH2-pre/post.
The introduction of functional groups at each step was monitored by IR spectroscopy. For nitro-containing materials, signals at 1534 and 1348 cm–1 were observed; these peaks disappeared after reduction with tin(II) chloride (Fig. 2, curves 1, 2). The IR spectra of aminated materials demonstrate signals in the range of 3300– 3500 cm–1 corresponding to N–H vibrations (Fig. 2, curve 3). Furthermore, complete cross-coupling was clearly evidenced by the lack of 1076 cm–1 peak (typical of C–Br bond) in the spectra of PAF-20–NO2-pre and PAF-30–NO2-pre.
The synthesized frameworks were examined by low-temperature nitrogen adsorption/desorption (Fig. 3). A steep rise in the low relative pressure region (P/P0 = 0–0.05) for all the PAFs indicates the presence of micropores in the frameworks. In the relative pressure region of 0.2–0.9, the adsorption isotherm gradually increases without reaching a plateau, and a hysteresis loop between the adsorption and desorption curves indicates the presence of mesopores. Another steep rise, between 0.9 and 1.0, serves as evidence of larger mesopores (20–30 nm) in the structure. For PAF-20–NH2-pre/post, the decrease in the specific surface area keeps within the instrumental error (2–5%). For the PAF-30–NH2-pre/post material, the decrease in SBET along with the change in the slope of the nitrogen adsorption curve is more pronounced in comparison with PAF-30–NO2-pre/post and can be explained by residual amounts of tin compounds that block the framework pores.
Data on the porous characteristics of the samples clearly show a decline both in the specific surface area and the total pore volume of the pre-modified PAFs after additional nitric acid treatment. This could be caused by the introduction of functional groups into the pre-generated porous structure (Table 1). On the other hand, the elemental analysis data clearly indicate that the nitrogen content in PAF-20–NH2-pre/post (5.1 wt %) is lower than in PAF-20–NH2-pre, a modified material prepared from tetrakis(3-amino-4-bromophenyl)methane monomer without any post treatment (6.0 wt %) [14]. This observation may serve as evidence that no additional nitro groups were introduced, and therefore the porosity decline was caused by framework degradation. In the case of PAF-30–NH2-pre/post, we observed an increased nitrogen content compared to the corresponding pre-modified framework, PAF-30–NH2-pre (5.9 wt % vs. 5.1 wt %, respectively), and a decreased proportion of large mesopores. This confirms the introduction of additional nitro groups into PAF-30–NO2-pre structure after nitric acid treatment.
Based on PAF-20–NH2-pre/post and PAF-30–NH2-pre/post, we synthesized palladium catalysts with nominal metal loads of 2 and 5 wt %. The particles were encapsulated into the support by its impregnation with a palladium (II) acetate solution in methylene chloride followed by metal reduction with sodium borohydride.
The elemental analysis shows that the nominal metal load of 2 wt % was actually achieved both for 2%Pd@PAF-20–NH2-pre/post and 2%Pd@PAF-30–NH2-pre/post. In contrast, the nominal 5 wt % Pd load was never reached, neither for 5%Pd@PAF-20–NH2-pre/post nor 5%Pd@PAF-30–NH2-pre/post. Moreover, in the case of 5%Pd@PAF-20–NH2-pre/post, the actual metal content proved to be similar to that in the 2%Pd counterpart, thus indicating a definite limitation of the adsorption capacity of PAF-20–NH2-pre/post. The actual Pd content in 5%Pd@PAF-30–NH2-pre/post, while somewhat higher (2.42 wt %), was also far from the nominal load. The reduction of limitations are most likely caused by a higher length of linkers between the tetraphenylmethane moieties in the framework, resulting in freer pore channels and less hindered diffusion of the metal salt.
The TEM micrographs clearly show that the concentration of the salt solution used in the synthesis has an effect on the Pd particle distribution and sizes (Fig. 4). Despite the similarity of the average particle size (about 2 nm) for all the catalysts, they clearly exhibit different size distribution histograms. 2%Pd@PAF-20–NH2-pre/post and 2%Pd@PAF-30–NH2-pre/post are characterized with distributions close to normal, with very small fraction of particles larger than 3 nm, thus confirming their incorporation into the framework pores (Figs. 4a and 4b). However, agglomerates of small particles were present on the PAF-30–NH2-pre/post surface, whereas no particle agglomerates were detected on the framework surface in 2%Pd@PAF-20–NH2-pre/post.
In the cases of 5%Pd@PAF-20–NH2-pre/post and 5%Pd@PAF-30–NH2-pre/post, the Pd reduction from the more concentrated solution markedly increased the fraction of 0.5–1.5 nm particles. This can be explained by the fact that the more concentrated solution filled more efficiently small pores located deeper in the support particles (Fig. 4c and 4d). Furthermore, a portion of the metal remaining on the surface caused the presence of large particles (up to 4.5–5 nm) and agglomerates of small particles (see the micrographs).
Catalytic Tests
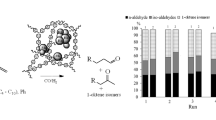
The synthesized catalysts were tested in the selective hydrogenation of C6 and C8 acetylenes and dienes. These hydrocarbons are contained in a pyrocondensate (a distillate with a boiling range of 36–204°C produced in thermal, steam, or catalytic cracking of naphtha) and act as catalytic poisons in polymerization processes. Specifically, 1-hexyne, 1-octyne, phenylacetylene (terminal alkynes), 4-octyne (an alkyne with an unsaturated bond in the carbon chain), isoprene, and 2,5-dimethylhexadiene-2,4 (conjugated dienes differing in chain lengths) were used as substrates. All reactions were carried out without solvents. Table 2 presents the proportions of hydrogenation products obtained from the substrates tested in the study.
Selective hydrogenation of unsaturated compounds is known to be a structure-sensitive reaction; therefore, we found it reasonable to investigate a series of substrates with different structures to gain better insight into the catalyst structure [17, 26]. The activity of the catalytic systems synthesized in our study was anticipated to depend on a variety of parameters such as the porous properties of the material, the size and arrangement of metal particles, and the number of functional groups. Bearing in mind that amino groups are largely located in benzene rings near the cores of tetraphenylmethane moieties—and assuming that, during impregnation of the materials, metal ions were mostly coordinated along these amino groups, resulting in generation of nanoparticles in these locations—it would be fair to expect additional steric constraints on the framework structure and an increased influence of the amino groups on the electronic parameters and geometry of active sites.
All the catalyst samples exhibited the highest activity in the hydrogenation of dienes (i.e., isoprene and 2,5-dimethylhexadiene-2,4) and 4-octyne. A 100% conversion of these substrates was achieved as early as after 30 min, though this was accompanied by relatively low selectivity towards the main products (methylbutenes, 2,5-dimethylhexene-2, and 4-octene, respectively). The rapidness of the reaction was most probably associated with hindered diffusion of the resultant olefins from metal nanoparticles in view of the steric constraints of the porous structure.
In the hydrogenation of 4-octyne, the main product was 4-octene due to its highest thermodynamic stability; high concentrations of octane are also worth noting. Isoprene was mainly converted to 2-methylbutene-2, the most thermodynamically stable compound under the reaction conditions imposed. The product ratios obtained for isoprene hydrogenation enabled us to comparatively assess the activity of all the catalysts. The highest activity was achieved by 5%Pd@PAF-20–NH2-pre/post, which mainly promoted the production of 2-methylbutane, presumably due to two factors: (i) this catalyst mostly consists of small palladium particles (<2 nm), which are characterized by a higher content of coordinatively unsaturated atoms that promote hydrogenation up to alkane; and (ii) the smaller pore size and dense arrangement of particles in PAF-20–NH2-pre/post hinder the diffusion of the resultant olefin from the palladium surface, thus facilitating its further conversion to alkane.
Despite essentially equal metal concentrations in 2%Pd@PAF-20–NH2-pre/post and 5%Pd@PAF-20–NH2-pre/post, the activity of the 2%Pd sample proved to be markedly lower due to a smaller fraction and looser distribution of sub-2 nm particles, thus facilitating the diffusion of the resultant alkenes from the palladium’s active sites. For the same reason, 2%Pd@PAF-30–NH2-pre/post exhibited a lower activity than 2%Pd@PAF-20–NH2-pre/post. Despite the similar concentrations and size distributions of metal particles in these catalysts, the former has a less hindered space around the particles. It should also be noted that 5%Pd@PAF-30–NH2-pre/post exhibited no high activity, despite almost doubling the Pd content in this catalyst. In all likelihood, its sub-2 nm particles effectively filled the pore space, making this catalyst comparable to the other samples in terms of diffusion properties.
In 2,5-dimethylhexadiene-2,4 hydrogenation, the product proportions were almost identical for all the catalysts. Due to the steric hindrances, this substrate reacted more slowly than isoprene; as a result, the main product was 2,5-dimethylhexene-2, with its isomer (2,5-dimethylhexene-3) accounting for 16–21%, and the saturated product (2,5-dimethylhexane) only for 4–5%.
In the case of terminal alkynes, namely 1-hexyne and 1-octyne, the opposite trend was observed. All the catalysts, except for 5%Pd@PAF-20–NH2-pre/post in 1-octyne hydrogenation, exhibited relatively low activity. In all likelihood, the diffusion of these substrates to active sites is less hindered due to their linear structure. Moreover, their complexation with the surface of Pd nanoparticles makes a significant contribution [27, 28]: this process is hindered for 4-octyne due to steric limitations. The catalytic activity in the hydrogenation of 1-octyne proved to be higher than that for 1-hexyne; this is due to the presence of a longer hydrocarbon substituent adjacent to the triple bond, resulting in the formation of a less stable complex. In the presence of 5%Pd@PAF-20–NH2-pre/post, 100% conversion was achieved, probably because the resultant 1-octene (more branched than 1-octyne) could not diffuse from active metal sites; therefore, the 1-octene was subject to isomerization followed by conversion to octane. This assumption is confirmed by the low activity of this catalyst in the hydrogenation of 1-hexyne.
Phenylacetylene fell between terminal and internal alkynes in terms of catalytic activity. On the one hand, the presence of a bulky benzene ring causes steric hindrances responsible for the lower hydrogenation activity of this substrate than that of 4-octyne and dienes. On the other hand, the weak M effect of the benzene ring may also hinder the adsorption of the substrate on the palladium surface. This precludes strong complexation with the metal [29, 30], unlike the cases of 1-hexyne and 1-octyne, thus preventing the catalyst from deactivation.
To investigate the influence of PAF structure on the hydrogenation parameters, for all the tested substrates the reactions were carried out at the same substrate to metal molar ratio of 20 000. For 2%Pd@PAF-20– NH2-pre/post and 5%Pd@PAF-20–NH2-pre/post, the effects of the substrate/Pd ratio on the catalytic activity and selectivity were demonstrated in the hydrogenation of 1-octyne (Table 3). At a ratio of 20 000, the substrate conversion on 2%Pd@PAF-20–NH2-pre/post was 34%; however, the decrease of the ratio to 10 000 abruptly boosted the conversion to 83%, with the 1-octene selectivity remaining at about 90%. For 5%Pd@PAF-20–NH2pre/post, increasing the substrate/Pd ratio from 20 000 to 40 000 enabled us to suppress the side reactions caused by hindered diffusion in the pores. As a result, the 1-octene selectivity reached 97% despite the substrate conversion drop to 30%.
For all the catalysts synthesized, the reusability was investigated (Table 4). In the hydrogenation of 4-octyne, all catalysts retained high activity after six consecutive reaction cycles, with the selectivity towards octenes remaining at 75–85% and the product ratios still matching those observed in the first cycle. For 2%Pd@PAF-20–NH2-pre/post and 2%Pd@PAF-30–NH2-pre/post, we further examined the reusability in phenylacetylene hydrogenation (Table 4). The conversion of this substrate rapidly declined cycle by cycle, while the selectivity remained fairly high (92–93%). This pattern correlates with our assumption of gradual adsorption of terminal alkynes on the Pd surface: due to the steric constraints and –M effect of the benzene ring, the adsorption of phenylacetylene was slower than that of 1-hexyne and 1-octyne.
Finally, we evaluated the activity and selectivity of the catalysts synthesized in this study in comparison with the Pd catalytic systems described in previous public reports (Table 5). In a series of previous studies performed by our research team, a pair of catalysts derived from pre-modified amino-containing PAFs synthesized from tetrakis(3-amino-4-bromophenyl)methane without further post-treatment (labeled Pd-PAF-20–NH2-pre and Pd-PAF-30–NH2-pre) were tested in selective hydrogenation [14]. The catalysts prepared in the present work under identical conditions exhibited higher activity while maintaining styrene selectivity at 92–93%. The structure of PAF-20–NH2-pre/post and PAF-30–NH2-pre/post is beneficial for the generation of particles about 2 nm in size, which provide high conversions, and the high content of amino groups allows selectivity above 90%. The systems synthesized in this study proved to be more active than industrial Lindlar catalysts, and more selective than commercially available Pd/C catalysts. Importantly, the catalysts under study were investigated at markedly lower substrate/metal ratios and under milder conditions. It is fair to consider the amino-containing PAFs to be related to catalysts based on cross-linked dendrimer matrices. The meso-G3-DMDPDI-Pd catalyst studied in [31] exhibited higher activity, with the selectivity comparable to the values achieved by our systems. Furthermore, this catalytic system was distinguished by a higher metal concentration.
CONCLUSIONS
Two porous aromatic frameworks, designated as PAF-20–NH2-pre/post and PAF-30–NH2-pre/post, were synthesized using a combination of pre- and post-modification. In the case of the PAF-20 series, this approach proved to be less effective because of the deactivating impact from nitro groups. These PAFs were used to synthesize catalysts with various palladium concentrations: 2%Pd@PAF-20–NH2-pre/post, 2%Pd@PAF-30–NH2-pre/post, 5%Pd@PAF-20–NH2-pre/post, and 5%Pd@PAF-30–NH2-pre/post. It was shown that, after impregnation with a more concentrated metal salt solution, Pd particles fill the small pores located deeper in the grain of the support more effectively. All of the synthesized catalysts were tested in selective hydrogenation of various alkynes and dienes, with 5%Pd@PAF-20–NH2-pre/post exhibiting the highest activity. Although all the catalysts showed low activity in the hydrogenation of terminal alkynes, the reaction occurred rapidly for dienes and internal alkynes because the resultant olefins, trapped in the pores, could not diffuse from active sites. All the catalysts withstood at least six successive reaction cycles when using 4-octyne; in the hydrogenation of phenylacetylene, gradual deactivation was observed.
REFERENCES
Tian, Y. and Zhu, G., Chem. Rev., 2020, vol. 120, no. 16, pp. 8934–8986. https://doi.org/10.1021/acs.chemrev.9b00687
Ben, T., Ren, H., Shengqian, M., Cao, D., Lan, J., Jing, X., Wang, W., Xu, J., Deng, F., Simmons, J.M., Qiu, S., and Zhu, G., Angew. Chemie Int. Ed., 2009, vol. 48, no. 50, pp. 9457–9460. https://doi.org/10.1002/anie.200904637
Hei, Z.-H., Huang, M.-H., Luo, Y., and Wang, Y., Polym. Chem., 2016, vol. 7, no. 4, pp. 770–774. https://doi.org/10.1039/C5PY01682G
Banerjee, D., Elsaidi, S.K., Aguila, B., Li, B., Kim, D., Schweiger, M.J., Kruger, A.A., Doonan, C.J., Ma, S., and Thallapally, P.K., Chem. – A Eur. J., 2016, vol. 22, no. 49, pp. 17581–17584. https://doi.org/10.1002/chem.201603908
Karakhanov, E.A., Boronoev, M.P., Subbotina, E.S., Zolotukhina, A.V., Maximov, A.L., and Filippova, T.Y., Petrol. Chem., 2016, vol. 56, no. 12, pp. 1114–1122. https://doi.org/10.1134/S0965544116120045
Karakhanov, E.A., Maksimov, A.L., Zolotukhina, A.V., Kardashev, S.V., and Filippova, T.Y., Petrol. Chem., 2012, vol. 52, no. 5, pp. 289–298. https://doi.org/10.1134/S0965544112050052
Nikolaev, S.A. and Krotova, I.N., Petrol. Chem., 2013, vol. 53, no. 6, pp. 394–400. https://doi.org/10.1134/S0965544113050071
Glotov, A., Vutolkina, A., Artemova, M., Demikhova, N., Smirnova, E., Roldugina, E., Stavitskaya, A., Ivanov, E., Egazar’yants, S., and Vinokurov, V., Appl. Catal. A: General, 2020, vol. 603, no. 2, p. 117764. https://doi.org/10.1016/j.apcata.2020.117764
Glotov, A., Vutolkina, A., Pimerzin, A., Nedolivko, V., Zasypalov, G., Stytsenko, V., Karakhanov, E., and Vinokurov, V., Catalysts, 2020, vol. 10, no. 5, pp. 7–9. https://doi.org/10.3390/catal10050537
Kulikov, L.A., Terenina, M.V., Kryazheva, I.Y., and Karakhanov, E.A., Petrol. Chem., 2017, vol. 57, no. 3, pp. 222–229. https://doi.org/10.1134/S0965544117020177
Jing, L.P., Sun, J.S., Sun, F., Chen, P., and Zhu, G., Chem. Sci., 2018, vol. 9, no. 14, pp. 3523–3530. https://doi.org/10.1039/c8sc00510a
Karakhanov, E., Maximov, A., Terenina, M., Vinokurov, V., Kulikov, L., Makeeva, D., and Glotov, A., Catal. Today, 2020, vol. 357, pp. 176–184. https://doi.org/10.1016/j.cattod.2019.05.028
Bazhenova, M., Kulikov, L.A., Bolnykh, Y.S., Maksimov, A.L., and Karakhanov, E.A., SSRN Electron. J., 2022, vol. 170, no. 7, p. 106486. https://doi.org/10.2139/ssrn.4079318
Makeeva, D., Kulikov, L., Zolotukhina, A., Maximov, A., and Karakhanov, E., Mol. Catal., 2022, vol. 517, p. 112012. https://doi.org/10.1016/j.mcat.2021.112012
Kulikov, L., Kalinina, M., Makeeva, D., Maximov, A., Kardasheva, Y., Terenina, M., and Karakhanov, E., Catalysts, 2020, vol. 10, no. 10, p. 1106. https://doi.org/10.3390/catal10101106
Barin, G., Peterson, G.W., Crocellà, V., Xu, J., Colwell, K.A., Nandy, A., Reimer, J.A., Bordiga, S., and Long, J.R., Chem. Sci., 2017, vol. 8, no. 6, pp. 4399–4409. https://doi.org/10.1039/c6sc05079d
Molnár, Á., Sárkány, A., and Varga, M., J. Mol. Catal. A: Chem., 2001, vol. 173, nos. 1–2, pp. 185–221. https://doi.org/10.1016/S1381-1169(01)00150-9
Crespo-Quesada, M., Cárdenas-Lizana, F., Dessimoz, A.-L., and Kiwi-Minsker, L., ACS Catal., 2012, vol. 2, no. 8, pp. 1773–1786. https://doi.org/10.1021/cs300284r
Melnikov, D., Stytsenko, V., Saveleva, E., Kotelev, M., Lyubimenko, V., Ivanov, E., Glotov, A., and Vinokurov, V., Catalysts, 2020, vol. 10, no. 6, p. 624. https://doi.org/10.3390/catal10060624
Nikolaev, S.A., Zanaveskin, L.N., Smirnov, V.V., Averyanov, V.A., and Zanaveskin, K.L., Russ. Chem. Rev., 2009, vol. 78, no. 3, pp. 231–247. https://doi.org/10.1070/rc2009v078n03abeh003893
Borodziński, A. and Bond, G.C., Catal. Rev., 2006, vol. 48, no. 2, pp. 91–144. https://doi.org/10.1080/01614940500364909
Lindlar, H., Helv. Chim. Acta, 1952, vol. 35, no. 2, pp. 446–450. https://doi.org/10.1002/hlca.19520350205
García-Mota, M., Gómez-Díaz, J., Novell-Leruth, G., Vargas-Fuentes, C., Bellarosa, L., Bridier, B., Pérez-Ramírez, J., and López, N., Theor. Chem. Acc., 2011, vol. 128, no. 4, pp. 663–673. https://doi.org/10.1007/s00214-010-0800-0
Witte, P.T., de Groen, M., de Rooij, R.M., Bakermans, P., Donkervoort, H.G., Berben, P.H., and Geus, J.W., Stud. Surf. Sci. Catal., 2010, vol. 175, pp. 135–143. https://doi.org/10.1016/S0167-2991(10)75017-5
Witte, P.T., Boland, S., Kirby, F., van Maanen, R., Bleeker, B.F., de Winter, D.A.M., Post, J.A., Geus, J.W., and Berben, P.H., ChemCatChem., 2013, vol. 5, no. 2, pp. 582–587. https://doi.org/10.1002/cctc.201200460
Jackson, S.D., Hamilton, C.A., Kelly, G.J., and De Bruin, D., React. Kinet. Catal. Lett., 2001, vol. 73, no. 1, pp. 77–82. https://doi.org/10.1023/A:1013924921651
He, G., Song, Y., Kang, X., and Chen, S., Electrochim. Acta, 2013, vol. 94, pp. 98–103. https://doi.org/10.1016/j.electacta.2013.01.134
Molnár, Á., Smith, G.V., and Bartók, M., J. Catal., 1986, vol. 101, no. 1, pp. 67–72. https://doi.org/10.1016/0021-9517(86)90229-0
Karakanov, E.A., Zolotukhina, A.V., Ivanov, A.O., and Maximov, A.L., ChemistryOpen, 2019, vol. 8, no. 3, pp. 358–381. https://doi.org/10.1002/open.201800280
Karakhanov, E.A., Maximov, A.L., Zakharyan, E.M., Zolotukhina, A.V., and Ivanov, A.O., Mol. Catal., 2017, vol. 440, pp. 107–119. https://doi.org/10.1016/j.mcat.2017.07.011
Karakhanov, E.A., Maximov, A.L., and Zolotukhina, A.V., Mol. Catal., 2019, vol. 469, pp. 98–110. https://doi.org/10.1016/j.mcat.2019.03.005
Jackson, S.D. and Shaw, L.A., Appl. Catal. A: General, 1996, vol. 134, no. 1, pp. 91–99. https://doi.org/10.1016/0926-860X(95)00194-8
Deng, D., Yang, Y., Gong, Y., Li, Y., Xu, X., and Wang, Y., Green Chem., 2013, vol. 15, no. 9, p. 2525. https://doi.org/10.1039/c3gc40779a
Funding
This study was carried out within the state assignment “Petrochemistry and Catalysis. Rational use of carbonaceous raw materials” (project no. 121031300092-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
A.L. Maximov, a co-author, is the Chief Editor at the Neftekhimiya (Petroleum Chemistry) Journal. The other co-authors declare no conflict of interest requiring disclosure in this article.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makeeva, D.A., Kulikov, L.A., Oskina, E.D. et al. Palladium Catalysts Based on Nitrogen-Containing Porous Aromatic Frameworks for Hydrogenation of Unsaturated Compounds. Pet. Chem. 62, 1183–1194 (2022). https://doi.org/10.1134/S0965544122090092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122090092