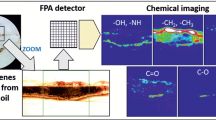
Abstract
Asphaltene deposition from crude oil, induced by n-heptane and acetone flows in a microfluidic device, was studied by optical and FTIR spectroscopic imaging techniques in situ. It was found that n-heptane and acetone penetrate crude oil in completely different ways, which is responsible for the formation of deposits with different structures and chemical compositions. The contact of the n-heptane flow with crude oil leads to fast aggregation of asphaltenes at the interface, resulting in the formation of a compact deposit fixed to the surface. Subsequent slow diffusion of n-heptane into crude oil causes asphaltene aggregation and precipitation of loose deposits. In the case of acetone, the flocculant diffuses (faster than n-heptane) into crude oil, which results in removal of its soluble components and in the formation of deposits appearing as strips as well. The distribution of the functional groups (–OH(NH), CH2–CH3, C=O, C–O, S=O) in the deposits is spatially heterogeneous. The asphaltenes deposits formed at the flocculant-crude oil interface are richer in the functional groups than those formed during diffusion. No carbonyl groups were revealed in the deposits formed in the acetone flow, while oxygen-containing groups such as –OH(NH) and S=O were present, which was an unusual result.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Asphaltenes are often compared to cholesterol [1], whose aggregation and following deposition causessimilar problems. Precipitation of asphaltenes together with other heavy components of crude oil, caused by changes in temperature or pressure or by mixing of different kind of crude oils, leads to contamination of the working surfaces of process facilities [2]. Mutually complementary information on the asphaltene aggregation and deposition can be revealed by comprehensive studies of the behavior of asphaltenes under exposure to various factors, both physical and chemical, highly efficient advanced physicochemical methods [3–5].
Microfluidics (microhydrodynamics) allows quickly capturing large amounts of data, in particular, automatically using small sample volumes [6]. Microfluidic technology holds promise for studies into enhanced oil recovery [7], asphaltenes deposition in porous media, separation of water droplets from the crude oil phase [8], selection of surfactants, and emulsification/demulsification processes in water-in-oil emulsions [9], as well as for examination of the rheological properties of crude oil [10].
Glass is the most used material for design of microfluidic devices, though transparent polymers are also applied for this purpose [11]. Glass microfluidic devices were employed to study the properties of asphaltenes, the onset point of asphaltenes precipitation [12, 13], deposition in the presence of chemical dispersants [14], as well as of metal oxide nanoparticles [15] and various types of ions [16], and for modeling hydrophilic porous rocks [17] and pore-throatsls [18]. Unfortunately, such materials are mostly transparent only to UV and visible light, which creates certain limitations in studies of the chemical composition of liquids. In a recent review dedicated to microfluidic systems employed for asphaltenes characterization only two cases of application of vibrational spectroscopy for this purpose were discussed [19].
Vibrational spectroscopy methods (FTIR and Raman spectroscopy) have proved their importance for determining the characteristics of crude oil and its components [20–24]. Owing to technical capabilities, they offer in terms of speed, precision, sample positioning, etc., these methods find application in microfluidics, as described in reviews [25, 26]. Technically, FTIR spectroscopic imaging differs from classical FTIR spectroscopy only in using a “matrix” detector, which allows obtaining spatial information about the sample. For studying the processes in microfluidic devices, the transmission and attenuated total internal reflection (ATR) modes can be employed, depending on the layout involved [27, 28]. Combined use of microfluidic device with the FTIR spectroscopic imaging method allowed monitoring direct reaction of isotopic H/D exchange between H2O and D2O when they have been mixed [29], as well as studying chemical reactions in two-phase systems [30], live cells [31], drug dissolution [32], polymerization processes for monomers [33], the imaging of the ionic liquids mixing [34].
The spatial distribution of compounds (asphaltenes, carbonates, sulfates, sulfoxides, oxalates, and possibly coke) in real crude oil deposits from heat exchanger was first shown by ATR-FTIR spectroscopic imaging [35]. More recently, this technique has been used to study in situ deposits formed in crude oil [36–38] and their blends as dependent on temperature [39] and CO2 pressure conditions [40].
Our earlier work [41] was the first to demonstrate the application of FTIR spectroscopic imaging using microfluidic device for in situ monitoring of n-heptane flow-induced precipitation and deposition of asphaltenes from benzene solution. In [42], the capabilities of the IR microscopy and FTIR spectroscopic imaging techniques in studying complex multicomponent systems were compared and analyzed. The deposits formed in the n-heptane flow from crude oil were investigated, and their spatial heterogeneity with respect to certain functional groups was revealed.
Light n-alkane is a commonly used nonpolar flocculant for the separation of asphaltene fractions according to the solubility classification. A more detailed study of the composition of the asphaltene fractions relies on the use of other nonpolar solvents such as diethyl and diisopropyl ethers and hexamethyldisiloxane [43], as well as of polar acetone [44–47]. It was shown [45] that, compared to the asphaltene subfractions precipitated by nonpolar flocculants, those obtained from polar flocculants exhibit major structural differences and a tendency to aggregate. Also, use of acetone leads to a nonmonotonic dependence of the molecular sizes of the asphaltene fractions, by contrast to the case of n-heptane [48].
A combination of microfluidics with an advanced FTIR spectroscopic imaging technique together with the use of different types of flocculants provides new insights into asphaltenes deposition regularities. In this study, we demonstrate the application of in situ FTIR spectroscopic imaging to monitoring n-heptane- and acetone-induced asphaltenes deposition from crude oil in the flow regime with the use of a microfluidic device.
EXPERIMENTAL
In the experiments crude oil was used with the following characteristics: density 0.936 g/cm3; content, wt %: aromatic hydrocarbons 41.9; aliphatic hydrocarbons 18.8; sulfur 2.2, nitrogen 0.38; asphaltenes 6.0; resins 8.8. n-Heptane and acetone (both for spectroscopy) were obtained from Komponent-Reaktiv (Russia) and used without further purification.
The experimental procedure employed for in situ FTIR spectroscopic imaging was described in detail in [41]. In the present study, a Bruker Vertex 70 V FTIR spectrometer equipped with an imaging macro chamber (IMAC) fitted with a 64×64 focal plane array detector was used. The microfluidic device was based on a liquid IR cell (Pike Technologies, the United States) with CaF2 optical windows. In view of a significantly higher viscosity of the crude oil relative to the solution of asphaltenes (5 wt %) in benzene, the shape of the channel used in [41] was changed to an expanded semicircular cavity.
The experiment on the formation of asphaltenes deposit from crude oil in a flow (n-heptane or acetone) consisted of the following. The cavity in the microfluidic device was filled with crude oil, as shown in Fig. 1 (left). The cell was placed in the IMAC, and the flocculant was injected into the microfluidic device at a rate of 1 mL/h (Fig. 1, middle). During passing the flocculant through the IR cell for 30–90 min, the FTIR spectroscopic images were recorded simultaneously. The deposits formed on the CaF2 window surface were dried for one day to remove the flocculants (Fig. 1, right); they were also recorded using a digital VIS camera. For details on the method of formation of deposits and on their study by the FTIR spectroscopy imaging, see [41, 42].
Scheme of the experiment on deposit formation in the flow regime in the IR cell channel: (left) crude oil is placed in the cavity of the channel; (middle) aggregation, precipitation, and deposition of asphaltenes on the IR cell window surface; and (right) dry asphaltenes deposits fixed on the window surface.
RESULTS AND DISCUSSION
Visualization of asphaltenes deposition from crude oil. First, we visualized asphaltenes precipitation in the flow regime using the digital VIS camera with the view of analyzing the experimental conditions and flocculant feed rate and selecting deposit locations suitable for FTIR spectroscopy imaging studies. Also, this allowed observing differences in the formation of asphaltenes deposits from crude oil between n-heptane and acetone.
Figure 2 presents the snapshots of the channel at different times after the start of flow of n-heptane and acetone flocculants. In both cases, the first snapshot shows crude oil in the channel, and the next or following snapshots that follow demonstrate changes at the interface between the flocculant flow and crude oil. The n-heptane and acetone flows cause formation of a deposit appearing as a dark strip at the interface between the flocculant and crude oil, clearly observable after several minutes. Further, n-heptane and acetone penetrate into crude oil, and deposits appearing as strips are formed. In the case of n-heptane, the dark strip at the interface between the flow and crude oil expands over time, and the area below the strip gets lighter, i.e., once the asphaltenes particles precipitate, the solution gets lighter.
In [15], an experiment on visualization of the asphaltenes precipitation from crude oil by n-heptane in glass micromodels was carried out. The dark areas were attributed to asphaltenes aggregation and deposition, and the lighter areas were attributed, to the deasphalted oil phase. Two types of asphaltenes deposits were detected. Asphaltenes deposition at the interface between n-heptane and oil and self-aggregation of the precipitated asphaltenes in oil were observed.
In the present experiment asphaltene deposit was revealed in the form of a strip at the interface between the n-heptane flow and crude oil. Formation of asphaltenes deposit of the second type were also observed when passing n-heptane through the microfluidic device for 90 min or longer. These regularities are similar to those observed in the asphaltenes precipitation from synthetic oil (solutions in toluene and benzene), as described in detail in [12, 41].
In the case of acetone, the flocculant diffuses into crude oil, washing out soluble components and forming deposits also in the form of a strip. After 30–35 min of flow, acetone completely diffuses into crude oil, and deposits are formed, whereas the asphaltenes precipitation leads to formation of deposits of the first type, i.e., appearing as strips. Comparison of the snapshots for n-heptane and acetone clearly reveals certain differences in the formation of asphaltenes deposits, which may be attributed to different rates of diffusion into crude oil. Through this visualization alone, it is challenging to understand how much faster the acetone diffuses into crude oil compared to that of n-heptane. In this situation, the FTIR spectroscopic imaging technique may be helpful.
In situ chemical imaging of asphaltenes precipitation. The FTIR spectroscopic imaging method provides information about the spatial distribution of various chemical components over measured area as a function of time based on the bands in the IR spectra. FTIR spectroscopic imaging allows simultaneous monitoring of the concentration and spatial distribution of all the components (crude oil and n-heptane, acetone) over time with a spatial resolution of few micrometers. Figure 3 shows the FTIR spectroscopic images of the crude oil-filled cavity at different times after the start of the n-heptane flow. The images of crude oil and n-heptane distribution were generated using spectral bands at 1650–1560 cm–1 (stretching vibrational mode of the aromatic C=C bond) and 1160–1120 cm–1 (stretching vibrational mode of C–C skeleton bond), respectively.
The image recorded prior to starting the n-heptane flow clearly demonstrates the crude oil profile. After the flow of n-heptane is fed into the microfluidic device, the concentration front of crude oil and n-heptane is blurred over time, that is, n-heptane diffuses into the volume.Already after 6 min of the experiment, formation of the asphaltenes deposit as a strip at the interface between the n-heptane flow and crude oil is observed. Although the band at 1650–1550 cm–1 was used for analyzing the spatial distribution of the crude oil, the same band served for analysis of the distribution of asphaltenes. This band has been applied in our previous works to tracking asphaltene precipitation from crude oils in static conditions [36, 37, 39]. This confirms that the bands observed in the FTIR spectroscopic images correspond to asphaltenes deposits. In this study, FTIR spectroscopic images were also plotted using the integrated absorbances of the band at 1750–1650 cm–1, associated with the stretching vibrations of the C=O bond of the carbonyl groups in asphaltenes. These images also demonstrate well-visible deposits, suggesting a fairly high concentration of the C=O groups.
The results obtained are indicative of n-heptane penetration into crude oil, leading to its partial removal from the cavity with formation of carbonyl-containing asphaltenes deposits appearing as strips. After 25 min of the experiment, the interface between crude oil and n-heptane becomes less certain, indicating n-heptane penetration into crude oil.
Figure 4 presents the FTIR images of the cavity filled with crude oil at different times after the start of acetone flow. The images obtained for the crude oil and acetone are based on the distribution of the intensities of the bands in the range of 1650–1560 and 1120–1070 cm–1 (–CH3 rocking vibrational mode of acetone), respectively. Starting from the first minute of the experiment, one can observe removal of crude oil from the cavity by the acetone flow and formation of strips of deposits, for which the corresponding images reveal the presence of the band at 1650–1550 cm–1, described of asphaltenes. Within 6 min of the experiment acetone removes crude oil from the FPA array detector area in the cavity of the microfluidic device.
Comparison of Figs. 3 and 4 reveals generally similar trends in the asphaltenes deposition under n-heptane and acetone flow, specifically, those to formation of deposits appearing as strips. However, acetone causes formation of deposits faster than n-heptane at the same flow rate; it diffuses faster into crude oil and removes it, and formation of asphaltenes deposits is observed. Unfortunately, the range of vibrations of the C=O bonds is overlapped by the absorption bands of acetone, which are much more intense than those of n-heptane.
Ex situ study of asphaltenes deposits. The method developed by us for deposition of asphaltenes in a flocculant flow onto the surface of an IR-transparent material (CaF2) enables studies by the FTIR spectroscopic imaging technique [41]. Asphaltene deposit formed in nonequilibrium conditions of a flocculant flow can be spatially inhomogeneous of chemical composition, which might provide significant data on the mechanism of the precipitation and deposition. Experiments were carried out repeatedly with both n-heptane and acetone in order to obtain deposits that are firmly fixed to the CaF2 window surface. Unfortunately, it was not always possible to obtain a well-fixed deposit with a thickness suitable for FTIR spectroscopic studies. Previously [42], crude oil deposits formed by the n-heptane flow were studied by the IR microscopy and FTIR spectroscopic imaging, and their spatial heterogeneity with respect to certain functional groups was revealed. Herein, we will briefly describe those results for the sake of comparison with the data obtained for the deposits formed under acetone flow.
Figure 5a presents the snapshot of the asphaltenes deposits formed from crude oil in the n-heptane flow, which are fixed to the CaF2 window surface, and Fig. 5b shows the area that has been examined by the FTIR spectroscopic imaging. The FTIR spectroscopic images presented in Fig. 5c were obtained using the integrated absorbances of the bands in the ranges of 3600–3100, 3000–2800, 1800–1650, 1200–1100, and 1100– 980 cm–1, associated with the stretching vibrations of the O–H(N–H), С–Н, C=O, C–O, and S=O bonds in hydroxyl (amino), aliphatic (CH3/CH2/CH), carbonyl, ether, and sulfoxide groups in the deposits, respectively.
Snapshots of the asphaltenes deposits and FTIR spectroscopic images of the spatial distribution of various functional groups in the asphaltenes deposits formed from crude oil by n-heptane flow: (a) snapshot of the asphaltenes deposits fixed to the CaF2 window, formed from crude oil under n-heptane flow, (b) area examined by the FTIR spectroscopic imaging, and (c) FTIR spectroscopic images of the distribution of the functional groups. Image size is 2.56 mm × 2.56 mm.
As shown by the in situ study above, spatially heterogeneous deposits appearing as strips were formed along the flow direction at the interface between n-heptane and crude oil. Deposits of this type are formed in the initial stage of the crude oil contact with the n-heptane flow. Formation of deposits closer to the channel walls is due to n-heptane diffusion into crude oil; above the line corresponding to the channel wall one can observe spots, which are essentially the residual oil that got between the PTFE spacer and the CaF2 window.
In view of the spatial distribution of the O–H(N–H), C–H, C=O, C–O, and S=O groups, the highest concentration of these groups is observed specifically in the deposits formed by the n-heptane flow. This suggests predominant formation of deposits rich in heteroatomic functional groups from crude oil in the n-heptane flow. That fact is also confirmed by the data on the asphaltenes deposition from benzene solution in n-heptane flow [41], as well as from benzene solution and crude oil under static conditions [36]. This also agrees with the reported concentration of the most polar components in the least soluble asphaltene subfraction [49].
Figure 6 shows the FTIR spectroscopic images of the asphaltene deposits formed from crude oil in the acetone flow. Given the spatial distribution of the aliphatic groups, the deposits are concentrated at two locations, but deposits in the form of strips with low content of aliphatic groups are observed as well. The O–H(N–H) groups are more uniformly distributed over the deposits. The sulfoxide (S=O) groups, on the contrary, are concentrated at locations characterized by high content of CH2, CH3 groups. Within the measurement accuracy, the lack of C=O groups in the deposits is in evidence. As mentioned in the Introduction, acetone finds very limited use as a flocculant for the precipitation of asphaltenes and other crude oil components.
The influence of acetone as a polar flocculant on the properties of fractionated asphaltenes was discussed in most detail in [45, 47]. Precipitation was effected by adding acetone portions to a solution of asphaltenes to obtain their subfractions. In the first fraction the oxygen content was low; in the next fractions it increased, but the sulfur and nitrogen contents did not change. In the present study we observe the absence of specifically C=O groups, while –OH and –(NH), as well as S=O groups are present in the deposits.
To conclude, we revealed that n-heptane and acetone penetrate crude oil in completely different ways, which is responsible for the formation of deposits with different structures. Fast diffusion of acetone into crude oil leads to removal of its soluble components and fast aggregation of asphaltenes with formation of deposits fixed to the surface. The contact of the n-heptane flow with crude oil also results in fast aggregation of the asphaltenes, followed by the formation of stable deposits, though at points of contact only. However, further the n-heptane flow does not remove crude oil; rather, it slowly diffuses therein, causing aggregation of asphaltenes and their subsequent precipitation and compaction. Thus, our study demonstrated for the first time that n-heptane and acetone flows produce different effects on crude oil and on the formation of asphaltene-containing deposits.
Detailed analysis of various types of asphaltenes deposits revealed structural heterogeneity with respect to various functional groups (–OH, –(HN), CH2–CH3, C=O, C–O, S=O). The highest concentration of these groups is observed specifically in the asphaltenes deposits formed under flow rather than during slow diffusion. It was found that the deposits formed by the acetone flow do not contain carbonyl groups, while oxygen-containing groups such as –OH(NH) and S=O are present, which turned out to be an unusual result.
REFERENCES
Kokal, S.L. and Sayegh, S.G., All Days, SPE, 1995. https://doi.org/10.2118/29787-MS.GJ
Coletti, F., Joshi, H.M., Macchietto, S., and Hewitt, G.F., in Crude Oil Fouling: Deposit Characterization, Measurements, and Modeling, Coletti, F. and Hewitt, G.F., Eds., Amsterdam: Elsevier, 2015, pp. 1–22.
Zheng, C., Zhu, M., Zareie, R., and Zhang, D., J. Pet. Sci. Eng., 2018, vol. 168, pp. 148–155. https://doi.org/10.1016/j.petrol.2018.05.002
Mart’yanov, O.N., Larichev, Yu.V., Morozov, E.V., Trukhan, S.N., and Kazaryan, S.G., Russ. Chem. Rev., 2017, vol. 86, no. 11, pp. 999–1023. https://doi.org/10.1070/RCR4742
Kök, M.V., Varfolomeev, M.A., and Nurgaliev, D.K., J. Pet. Sci. Eng., 2017, vol. 154, pp. 537–542. https://doi.org/10.1016/j.petrol.2016.12.018
Hessel, V., Löwe, H., and Schönfeld, F., Chem. Eng. Sci., 2005, vol. 60, pp. 2479–2501. https://doi.org/10.1016/j.ces.2004.11.033
Gogoi, S. and Gogoi, S.B., J. Pet. Explor. Prod. Technol., 2019, vol. 9, pp. 2263–2277. https://doi.org/10.1007/s13202-019-0610-4
Tian, Y.S., Yang, Z.Q., Thoroddsen, S.T., and Elsaadawy, E., J. Pet. Sci. Eng., 2021, vol. 208, p. 109720. https://doi.org/10.1016/j.petrol.2021.109720
Lin, Y.-J., Perrard, A., Biswal, S.L., Hill, R.M., and Trabelsi, S., Energy Fuels, 2018, vol. 32, pp. 4903–4910. https://doi.org/10.1021/acs.energyfuels.8b00249
Keshmiri, K., Huang, H., and Nazemifard, N., Fuel, 2019, vol. 239, pp. 841–851. https://doi.org/10.1016/j.fuel.2018.11.044
Lin, Y.-J., He, P., Tavakkoli, M., Mathew, N.T., Fatt, Y.Y., Chai, J.C., Goharzadeh, A., Vargas, F.M., and Biswal, S.L., Langmuir, 2016, vol. 32, pp. 8729–8734. https://doi.org/10.1021/acs.langmuir.6b02376
Doryani, H., Malayeri, M.R., and Riazi, M., Fuel, 2016, vol. 182, pp. 613–622. https://doi.org/10.1016/j.fuel.2016.06.004
Telmadarreie, A. and Trivedi, J., Energy Fuels, 2017, vol. 31, pp. 9067–9079. https://doi.org/10.1021/acs.energyfuels.7b01347
Lin, Y.-J., He, P., Tavakkoli, M., Mathew, N.T., Fatt, Y.Y., Chai, J.C., Goharzadeh, A., Vargas, F.M., and Biswal, S.L., Energy Fuels, 2017, vol. 31, pp. 11660–11668. https://doi.org/10.1021/acs.energyfuels.7b01827
Beheshti, E., Doryani, H., Malayeri, M.R., and Riazi, M., J. Pet. Sci. Eng., 2021, vol. 205, p. 108839. https://doi.org/10.1016/j.petrol.2021.108839
Doryani, H., Malayeri, M.R., and Riazi, M., J. Mol. Liq., 2018, vol. 258, pp. 124–132. https://doi.org/10.1016/j.molliq.2018.02.124
Saadat, M., Yang, J., Dudek, M., Øye, G., and Tsai, P.A., J. Pet. Sci. Eng., 2021, vol. 203, p. 108647. https://doi.org/10.1016/j.petrol.2021.108647
Onaka, Y. and Sato, K., J. Pet. Sci. Eng., 2021, vol. 207, pp. 109176. https://doi.org/10.1016/j.petrol.2021.109176
Mozaffari, S., Ghasemi, H., Tchoukov, P., Czarnecki, J., and Nazemifard, N., Energy Fuels, 2021, vol. 35, pp. 9080–9101. https://doi.org/10.1021/acs.energyfuels.1c00717
Kiefer, J. and Corsetti, S., Analytical Characterization Methods for Crude Oil and Related Products, Shukla, A.K., Ed., Hoboken, NJ, USA: John Wiley and Sons, 2018, pp. 245–269. https://doi.org/10.1002/9781119286325.ch9
Kozhevnikov, I.V., Nuzhdin, A.L., and Martyanov, O.N., J. Supercrit. Fluids, 2010, vol. 55, pp. 217–222. https://doi.org/10.1016/j.supflu.2010.08.009
Chibiryaev, A.M., Kozhevnikov, I.V., Shalygin, A.S., and Martyanov, O.N., Energy Fuels, 2018, vol. 32, pp. 2117–2127. https://doi.org/10.1021/acs.energyfuels.7b01630
Rakhmatullin, I.Z., Efimov, S.V., Tyurin, V.A., Al-Muntaser, A.A., Klimovitskii, A.E., Varfolomeev, M.A., and Klochkov, V.V., J. Pet. Sci. Eng., 2018, vol. 168, pp. 256–262. https://doi.org/10.1016/j.petrol.2018.05.011
Guzmán-Osorio, F., Domínguez-Rodríguez, V., Adams, R., Lobato-García, C., Guerrero-Peña, A., and Barajas-Hernández, J., Egypt. J. Pet., 2021, vol. 30, pp. 63–67. https://doi.org/10.1016/j.ejpe.2021.03.003
Perro, A., Lebourdon, G., Henry, S., Lecomte, S., Servant, L., and Marre, S., React. Chem. Eng., 2016, vol. 1, pp. 577–594. https://doi.org/10.1039/C6RE00127K
Ewing, A.V. and Kazarian, S.G., Spectrochim. Acta, Part A: Mol. Biomol. Spectrosc., 2018, vol. 197, pp. 10–29. https://doi.org/10.1016/j.saa.2017.12.055
Ricci, C., Chan, K.L.A., and Kazarian, S.G., Appl. Spectrosc., 2006, vol. 60, pp. 1013–1021. https://doi.org/10.1366/000370206778397434
Chan, K.L.A., Niu, X., de Mello, A.J., and Kazarian, S.G., Lab. Chip., 2010, vol. 10, p. 2170. https://doi.org/10.1039/c004246c
Chan, K.L.A., Gulati, S., Edel, J.B., de Mello, A.J., and Kazarian, S.G., Lab. Chip., 2009, vol. 9, p. 2909. https://doi.org/10.1039/b909573j
Chan, K.L.A. and Kazarian, S.G., Anal. Chem., 2012, vol. 84, pp. 4052–4056. https://doi.org/10.1021/ac300019m
Chan, K.L.A. and Kazarian, S.G., Analyst, 2013, vol. 138, p. 4040. https://doi.org/10.1039/c3an00327b
Ewing, A.V., Clarke, G.S., and Kazarian, S.G., Biomicrofluidics, 2016, vol. 10, p. 024125. https://doi.org/10.1063/1.4946867
Ryu, M., Kimber, J.A., Sato, T., Nakatani, R., Hayakawa, T., Romano, M., Pradere, C., Hovhannisyan, A.A., Kazarian, S.G., and Morikawa, J., Chem. Eng. J., 2017, vol. 324, pp. 259–265. https://doi.org/10.1016/j.cej.2017.05.001
Chan, K.L.A., Shalygin, A.S., Martyanov, O.N., Welton, T., and Kazarian, S.G., J. Mol. Liq., 2021, vol. 337, p. 116412. https://doi.org/10.1016/j.molliq.2021.116412
Tay, F.H. and Kazarian, S.G., Energy Fuels, 2009, vol. 23, pp. 4059–4067. https://doi.org/10.1021/ef900304v
Gabrienko, A.A., Lai, C.H., and Kazarian, S.G., Energy Fuels, 2014, vol. 28, pp. 964–971. https://doi.org/10.1021/ef402255c
Gabrienko, A.A., Subramani, V., Martyanov, O.N., and Kazarian, S.G., Adsorpt. Sci. Technol., 2014, vol. 32, pp. 243–255. https://doi.org/10.1260/0263-6174.32.4.243
Gabrienko, A.A., Morozov, E.V., Subramani, V., Martyanov, O.N., and Kazarian, S.G., J. Phys. Chem., C, 2015, vol. 119, pp. 2646–2660. https://doi.org/10.1021/jp511891f
Gabrienko, A.A., Martyanov, O.N., and Kazarian, S.G., Energy Fuels, 2015, vol. 29, pp. 7114–7123. https://doi.org/10.1021/acs.energyfuels.5b01880
Gabrienko, A.A., Martyanov, O.N., and Kazarian, S.G., Energy Fuels, 2016, vol. 30, pp. 4750–4757. https://doi.org/10.1021/acs.energyfuels.6b00718
Shalygin, A.S., Kozhevnikov, I.V., Kazarian, S.G., and Martyanov, O.N., J. Pet. Sci. Eng., 2019, vol. 181, p. 106205. https://doi.org/10.1016/j.petrol.2019.106205
Shalygin, A.S., Milovanov, E.S., Yakushkin, S.S., and Martyanov, O.N., Petrol. Chem., 2021, vol. 61, pp. 1011–1018. https://doi.org/10.1134/S0965544121090127
Ilyin, S., Arinina, M., Polyakova, M., Bondarenko, G., Konstantinov, I., Kulichikhin, V., and Malkin, A., J. Pet. Sci. Eng., 2016, vol. 147, pp. 211–217. doi:10.1016/j.petrol.2016.06.020
Acevedo, S., Escobar, G., Ranaudo, M.A., Piñate, J., Amorín, A., Díaz, M., and Silva, P., Energy Fuels, 1997, vol. 11, pp. 774–778. https://doi.org/10.1021/ef960089n
Buenrostro-Gonzalez, E., Andersen, S.I., GarciaMartinez, J.A., and Lira-Galeana, C., Energy Fuels, 2002, vol. 16, pp. 732–741. https://doi.org/10.1021/ef0102317
Petrova, L.M., Abbakumova, N.A., Zaidullin, I.M., and Borisov, D.N., Petrol. Chem., 2013, vol. 53, pp. 81–86. https://doi.org/10.1134/S0965544113020084
Morantes, L.R., Percebom, A.M., and MejíaOspino, E., Fuel, 2019, vol. 241, pp. 542–549. https://doi.org/10.1016/j.fuel.2018.12.028
Buch, L., Fuel, 2003, vol. 82, pp. 1075–1084. https://doi.org/10.1016/S0016-2361(03)00006-1
Gawrys, K.L., Matthew Spiecker, P., and Kilpatrick, P.K., Pet. Sci. Technol., 2003, vol. 21, pp. 461–489. https://doi.org/10.1081/LFT-120018533
ACKNOWLEDGMENTS
A.S. Shalygin and E.P. Kovalev thank the Russian Science Foundation for financial support (project no. 20-79-00183, 2020 Competition for projects for the event “Conducting Initiative Research by Young Scientists” of the Presidential Program of Research Projects Implemented by Leading Scientists, Including Young Scientists) and the National Center for Catalyst Research, Collective Use Center, for the provided equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shalygin, A.S., Milovanov, E.S., Kovalev, E.P. et al. In Situ FTIR Spectroscopic Imaging of Asphaltene Deposition from Crude Oil under n-Heptane and Acetone Flows. Pet. Chem. 62, 1087–1095 (2022). https://doi.org/10.1134/S0965544122090079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122090079