Abstract
Conversion of mixtures of fuel oil with iron-containing substrates (carbon adsorbent and lignin, both modified with 0.5 wt % Fe) to hydrocarbon products and hydrogen was studied. The use of microwave radiation in the presence of the above-indicated iron-containing substrates capable of its absorption with the generation of breakdown phenomena and plasma is a promising approach to rapid processing of stable organic compounds of petroleum and natural origin into hydrocarbon products used in organic synthesis and for production of fuel components.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Most of oil fields in Eurasia are characterized by increased content of high-boiling fractions such as fuel oil and tar, sometimes reaching 40% [1, 2]. These fractions are enriched in virtually unprocessable asphaltenes and are characterized by increased concentrations of sulfur- and nitrogen-containing heteroatomic compounds. High chemical stability of high-molecular-mass compounds and increased content of heteroatomic compounds, which are often catalytic poisons, practically do not allow these compounds to be used as a feedstock for producing fuel components. The release of large amounts of toxic compounds also significantly complicates the development of procedures for their processing. On the other hand, high content of virtually unprocessable raw material complicates the development of efficient methods for producing the main power carriers, fuel components.
Increased attention is paid today to the development of processes for microwave treatment of many stable organic substrates [3–6]. As reported in [6, 7], the action of microwave radiation (MWR) on shale oil in the presence of iron oxide particles led to an increase in the yield of light hydrocarbons. In [8, 9], we developed procedures for rapid degradation of analogs of pollutants and toxic compounds, preliminarily adsorbed onto a porous carbon support capable of efficient absorption of microwave radiation. Further development of this method [10–14] consisted in finding optimum conditions for rapid conversion of highly stable substrates of petroleum and vegetable origin, such as methane, tar, and lignin of wood origin, to syngas, hydrogen, and liquid hydrocarbon products. The main aspect of these studies was the development of catalytic systems with high ability to absorb MWR. The MWR absorption level is characterized by the dielectric loss tangent [15]. At high values of this parameter, the irradiation gives rise to local electrical breakdown effects (BEs) (so-called “hot points”) and to plasma generation. It has also been shown [16] that the configuration of pores and concentration of iron-containing catalytic components also influence the MWR absorption. As found in [16], in macropores containing iron oxide nanoparticles the emergence of breakdown effects and plasma generation occur considerably faster. In conversion of lignin of wood origin, whose ability for MWR absorption is relatively low, deposition of up to 0.5 wt % iron oxide onto the surface led to a considerable increase in the heating rate, to the emergence of BEs, and to plasma generation. Iron-containing lignin can be readily converted to syngas by carbon dioxide reforming with up to 65% conversion of the organic mass [16]. The iron-containing carbon residue after the lignin conversion also exhibits high ability to absorb MWR. The iron-containing systems on a carbon support and the lignin processing residue were used for the conversion of tar to a broad fraction of hydrocarbons [13].
This study deals with the conversion of fuel oil in the presence of a carbon adsorbent modified with iron acetylacetonate (~0.5 wt % Fe) and of a mixture of fuel oil with lignin modified with ~0.5 wt % Fe to hydrocarbon products.
EXPERIMENTAL
Fuel oil was provided by Gazpromneft—Moscow Oil Refinery (Russia). Its boiling start point was 460°С, and the elemental composition (wt %) was as follows: С 85.5, Н 12.7, and S <1. Lignin of wood origin was provided by Kirov Biochemical Plant (Russia); its composition and physicochemical characteristics are given in [10, 11].
Iron-containing components were deposited onto the surface of the carbon adsorbent and lignin by impregnation with a benzene solution of iron acetylacetonate Fe(acac)3, taken in an amount corresponding to the moisture capacity. Prior to impregnation, the carbon adsorbent and lignin were kept in a vacuum oven for 5 h at 60°С to remove moisture. The calculated amount of a benzene solution of iron acetylacetonate was applied dropwise onto the surface of the substrates with stirring. The moisture capacity of lignin and carbon adsorbent was 4 and 5 cm3/g, respectively. After the adsorption of iron acetylacetonate, the substrates were dried at room temperature for 24 h and then heated in an argon stream at 50, 100, and 150°С for 2 h at each temperature. The iron content determined by atomic absorption spectrometry was 0.45 wt % on the carbon adsorbent and 0.48 wt % on lignin.
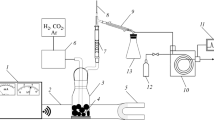
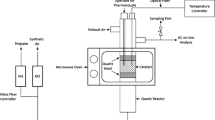
Experiments on fuel oil conversion in a mixture with iron-containing substrates (lignin or carbon adsorbent) containing 0.5 wt % Fe were performed on a novel laboratory microwave installation [10–13] in a quartz reactor (V = 50 cm3) in an argon stream 60 (cm3/min); the reaction mixture was subjected to the action of a traveling MWR wave (frequency 2.45 GHz).
In a typical experiment, the reactor was charged with fuel oil mixed with the iron-containing substrate; the substrate to fuel oil weight ratio was 1/8 or 1/4. The temperature in the reaction volume was measured with a thermocouple placed in an insulated heat-resistant housing with a hole on the level of the thermocouple end.
When switching on the magnetron, the traveling MWR wave in the reactor interacts with the loaded system capable to absorb the radiation, the temperature rapidly grows, breakdown effects arise, and plasma is generated. The temperature is controlled by the current feeding the magnetron.
The stability of the temperature conditions in the course of processing is ensured by the current feeding the magnetron and by the formation of a high concentration of vaporous products formed by cleavage of fuel oil and its mixture with lignin. The experiments were performed at the irradiation-induced temperature in the range 450–500°С. The vapors of the feed degradation products were fed into a dephlegmator and a condenser; in the latter, the liquid fraction was separated from the gaseous fraction. The gaseous products were fed to a gas meter and to chromatographic analysis. The experiment completion was characterized by the cessation of the evolution of product vapors and by rapid growth of temperature in the reaction zone.
The liquid isolated after the experiment was distilled to obtain the following fractions: s.b.–220°С, 220–350°С, and residue boiling above 350°С.
Gaseous reaction products were analyzed by gas chromatography with a CRYSTALLUX-4000M device (META-CHROM, Russia) using a 1.5 m long column packed with 0.5 mm α-Al2O3 granules bearing 15% squalane. A flame ionization detector was used. The carrier gas was Не (grade 6.0, Ballongaz, Russia). The amounts of Н2, СН4, СО, and СО2 were determined using a column packed with SKT carbon phase (META-CHROM) and a thermal conductivity detector; the carrier gas was argon (VCh 4.8 grade, Ballongaz).
The feed conversion was estimated from the weight loss; the agreement with the weight of the products taken off was reasonable (~10% discrepancy):
where Х is the feed conversion, %; Mf, feed weight, g; Mr, weight of the solid residue in the reactor after the experiment, g; and Mp, weight of products.
The liquid fractions obtained from fuel oil and lignin and taken off in the course the conversion were analyzed with a Leco Pegasus® BT 4D two-dimensional gas chromatograph/time-of-flight mass spectrometer (GC×GC-TOFMS). The device includes an Agilent 7890A gas chromatograph with the built-in second furnace and two-step cryomodulator and a Leco Pegasus® BT 4D time-of-flight mass analyzer. The series-connected columns [column 1: Rxi-5Sil phase (30 m × 0.25 mm × 0.25 μm); column 2: Rxi-17Sil phase (1.7 m × 0.10 mm × 0.10 μm)] ensure simultaneous separation of the main classes of organic compounds due to a combination of polar and nonpolar chromatographic phases.
Separation conditions: carrier gas helium, flow rate through the column 1 mL/min, flow split ratio 1 : 500, injector (septa) purging rate 3 mL/min; injector temperature 300°С; temperature schedule of the first furnace: initial temperature 40°С (2 min), then heating at a rate of 3°C/min to 320°C, and keeping at the final temperature for 5 min; the temperature of the second furnace and modulator is maintained on the level 6 and 21°С higher than that of the first furnace, respectively; modulation time on the modulator 6 s.
Mass spectrometer operation conditions: electron ionization (70 eV), ion source temperature 280°С, range of detected masses 35–520, recording rate 100 spectra per second. The analysis results were processed using CromaTOF software (Leco).
The composition of the high-boiling (≥350°C) residue from distillation of the liquid product was estimated by NMR. The 1Н NMR spectra were recorded with a Bruker AVANCE III HD spectrometer (400 MHz); the sample was dissolved in deuterochloroform.
RESULTS AND DISCUSSION
The iron-containing catalyst on carbon support has abnormally high dielectric loss tangent (~12.7) and, correspondingly, extremely high ability to absorb MWR [17]. The dynamics of microwave heating of the iron-containing systems under consideration is shown in Fig. 1 (curves 1, 2). As can be seen, lignin of wood origin does not exhibit sufficient ability to absorb MWR. On the other hand, after the deposition of ~0.5 wt % iron-containing active component onto its surface, as in the case of the iron-containing catalyst on a carbon support, MWR causes rapid heating, emergence of breakdown effects (BEs), and plasma generation.
Temperature profiles (heating dynamics) of the reaction zone: (1) FexOy/C containing ~0.5 wt % iron oxide; (2) lignin containing ~0.5 wt % iron-containing component; (3) initial lignin; (4) conversion of a mixture of fuel oil with Fe/C; (5) mixture of fuel oil with lignin containing ~0.5 wt % iron-containing component.
In irradiation of fuel oil containing the iron–carbon catalytic system and of fuel oil mixed with lignin containing ~0.5% iron-containing component, the temperature sharply increases, and the organic mass undergoes intense conversion with the formation of gaseous and liquid products. The time of complete processing of fuel oil is 20 min in joint conversion with FexOy/C (Fig. 1, curve 4) and 35 min when adding lignin modified with the iron-containing component (Fig. 1, curve 5).
The constant temperature of the reaction zone, varying in the range 450–500°С, in the course of the process is ensured by the current feeding the magnetron and by the formation of high concentration of vapor in cleavage of fuel oil and its mixture with lignin.
After the processing of a mixture of fuel oil with lignin is complete, the temperature sharply increases and corresponds to the heating dynamics shown in Fig. 1.
The balance of conversion products is shown in Table 1. As can be seen, fuel oil degrades with the predominant formation of liquid products.
The fuel oil conversion in the presence of an iron–carbon catalyst reaches 93% in 25 min of irradiation. In processing of a mixture of fuel oil with iron-containing lignin, the feed conversion is 76.8%. The fractional composition of products formed in processing of a mixture of fuel oil with lignin shows that, in the first step of processing, the fraction boiling above 350°С is formed in a large amount.
The composition of the gaseous products formed is shown in Table 2.
An important feature of the conversion of highly stable organic substrates in an inert atmosphere under MWR conditions is the formation of unsaturated gaseous С2 and С3 hydrocarbons and relatively high hydrogen concentration in the gas.
The 1H NMR spectrum recorded for the high-boiling fraction (Tb ≥ 350°С) obtained by processing a mixture of lignin and fuel oil contains signals corresponding to protons of aromatic systems (7–8 ppm), benzyl protons of aliphatic substituents in aromatic compounds (2–3 ppm), and protons of aliphatic compounds or substituents (0.7–2 ppm). A weak signal near 5.7 ppm is also observed, suggesting the presence of substituents with double bonds. Comparison of the integral intensities of signals of methyl (up to 1 ppm) and methylene (1–2 ppm) groups demonstrates the presence of a large amount of relatively short-chain and/or strongly branched molecular fragments. Comparison of the 1H NMR spectra of products obtained by processing of fuel oil with lignin and of the similar fraction isolated from products of fuel oil processing in the presence of the Fe/C system shows that they are virtually fully identical.
As shown previously, the irradiation of compounds exhibiting high ability to absorb MWR leads to the intensification of surface breakdown effects with the subsequent plasma generation [9]. Presumably, under the breakdown and plasma conditions, the highly stable С–Н bond undergoes strong polarization followed by cleavage. For example, as shown in [12], irradiation of methane in the presence of a catalytic system exhibiting high ability to absorb MWR leads to direct decomposition of methane to hydrogen and carbon.
The group composition of liquid products, calculated from data of two-dimensional gas chromatography/mass spectrometry (GC/MS), is given in Table 3. The results obtained show that the fuel oil conversion yields a wide range of hydrocarbons. Alkanes, olefins, and aromatic hydrocarbons are formed in the largest amounts; a relatively small amount of oxygen-containing cyclic products is also formed. It should also be noted the processing of a mixture of fuel oil with lignin yields noticeable amounts of decalin and diene hydrocarbons.
As noted in [11], lignin containing 0.5 wt % iron oxide nanoclusters has two functions: It absorbs MWR with the generation of breakdown effects and plasma and simultaneously acts as a carbon-containing feedstock. As seen from Fig. 1, deposition of ~0.5 wt % Fe leads to a nonadditive increase in the ability of iron-containing lignin to absorb microwave radiation, to intensification of breakdown effects, and to plasma generation. As shown previously by transmission electron microscopy (TEM) [18], under microwave irradiation iron acetylacetonate partially decomposes already in the application step and completely transforms into iron oxide nanoclusters with the formation of nanoparticles of 3–5 nm size, uniformly distributed on the surface, in the first minutes of the irradiation. The Mössbauer spectra recorded in the same study show that iron oxide particles are bonded to the surface oxygen of lignin [18].
To increase the yield of lower-boiling liquid products, the fraction boiling above 350°С, obtained by joint processing of fuel oil with lignin, was repeatedly exposed to MWR in the presence of the Fe/C catalyst. The conversion of the fraction boiling above 350°С under the action of MWR was 52.3% with the formation of 1.7% gaseous products and 50.6% liquid products boiling at temperatures of up to 350°С. As seen from Table 3, the mixture formed from the higher-molecular-mass fraction has the composition similar to that of the hydrocarbon-containing products obtained from fuel oil.
The polymeric structure of lignin is more resistant to degradation compared to hydrocarbon components of tar [13] and, as shown in this study, to those of fuel oil.
The temperature profile of the reaction zone (Fig. 1) shows that, in microwave irradiation of fuel oil in the presence of Fe/C (4) and of a mixture of fuel oil with lignin containing ~0.5 wt % iron oxide (5), the fuel oil conversion occurs considerably faster in the former case than in the latter case. Analysis of the products formed and comparison of their composition with that of the products obtained previously by conversion of iron-containing lignin taken alone suggest that the components mainly undergoing conversion are fuel oil hydrocarbons, whereas the major products released from lignin are hydrogen and carbon oxides [10, 11]. Another main function of iron-containing lignin is increased ability to absorb MWR.
The presence of metals in the residual fractions is one of factors complicating the fuel oil processing by traditional catalytic methods. Therefore, we analyzed for metals the solid carbon-containing residue from fuel oil processing. The metals recovered from fuel oil and present in the carbon-containing residue from joint processing of fuel oil with lignin are given in Table 4.
The results obtained show that the use of MWR in the presence of catalytic systems capable of its absorption with the generation of breakdown effects and plasma is a promising approach to rapid processing of stable organic substrates of petroleum and natural origin into hydrocarbon products used in organic synthesis and for production of fuel components.
REFERENCES
Sokolov, V.A., Bestupov, M.A., and Tikhomolova, T.V., Khimicheskii sostav neftei i prirodnykh gazov v svyazi c ikh proiskhozhdeniem (Chemical Composition of Crude Oils and Natural Gases in Connection with Their Origin), Moscow: Nedra, 1972.
Gordadze, G.N., Uglevodorody v neftyanoi geokhimii. Teoriya i praktika (Hydrocarbons in Petroleum Geochemistry. Theory and Practice), Moscow: Ross. Gos. Univ. Nefti i Gaza im. I.M. Gubkina, 2015.
Mutyala, S., Fairbridge, C., Paré, J.J., Bélanger, J.M., Ng, S., and Hawkins, R., Fuel Process. Technol., 2010, vol. 91, no. 2, pp. 127–135. https://doi.org/10.1016/j.fuproc.2009.09.009
Kim, T., Lee, J., and Lee, K.H., Carbon Lett., 2014, vol. 15, no. 1, pp. 15–24. https://doi.org/10.5714/CL.2014.15.1.015
Motasemi, F. and Afzal, M.T., Renew. Sustain. Energy Rev., 2013, vol. 28, pp. 317–330. https://doi.org/10.1016/j.rser.2013.08.008
Taheri-Shakib, J. and Kantzas, A., Fuel, 2021, vol. 305, article 121519. https://doi.org/10.1016/j.fuel.2021.121519
Zhu, J., Yang, Z., Li, X., Qi, S., and Jia, M., Energy Sci. Eng., 2018, vol. 6, no. 5, pp. 548–562. https://doi.org/10.1002/ese3.231
Tsodikov, M.V., Perederii, M.A., Chistyakov, A.V., Konstantinov, G.I., and Martynov, B.I., Solid Fuel Chem., 2012, vol. 45, no. 1, pp. 37–44. https://doi.org/10.3103/S0361521912010132
Tsodikov, M.V., Konstantinov, G.I., Chistyakov, A.V., Arapova, O.V., and Perederii, M.A., Chem. Eng. J., 2016, vol. 292, pp. 315–320. https://doi.org/10.1016/j.cej.2016.02.028
Tsodikov, M.V., Ellert, O.G., Vasil’kov, A.Yu., Nikolaev, S.A., Arapova, O.V., Konstantinov, G.I., and Bukhtenko, O.V., Chem. Eng. J., 2017, vol. 309, pp. 628–637. https://doi.org/10.1016/j.cej.2016.10.031
Arapova, O.V., Tsodikov, M.V., Chistyakov, A.V., and Konstantinov, G.I., Chem. Eng. Trans., 2017, vol. 57, pp. 223–228. https://doi.org/10.3303/CET1757038
Tsodikov, M.V., Ellert, O.G., Nikolaev, S.A., Arapova, O.V., Konstantinov, G.I., Bukhtenko, O.V., and Vasil’kov, A.Y., Chem. Eng. J., 2017, vol. 309, pp. 628–637.
Tsodikov, M.V., Chistyakov, A.V., Konstantinov, G.I., Borisov, R.S., Bondarenko, G.N., and Arapova, O.V., Petrol. Chem., 2021, vol. 61, pp. 721–728. https://doi.org/10.1134/S0965544121070070]
Chistyakov, A.V., Konstantinov, G.I., Tsodikov, M.V., and Maximov, A.L., Dokl. Phys. Chem., 2021, vol. 498, pp. 49–54. https://doi.org/10.1134/S0012501621050018
Durka, T., Van Gerven, T., and Stankiewicz, A., Chem. Eng. Techn.: Ind. Chem.–Plant Equip.–Process Eng.–Biotechnol., 2009, vol. 32, no. 9, pp. 1301–1312. https://doi.org/10.1002/ceat.200900207
Chistyakov, A.V., Konstantinov, G.I., Arapova, O.V., and Tsodikov, M.V., Chem. Eng. Trans., 2019, vol. 74, pp. 49–54. https://doi.org/10.3303/CET1974009
Tsodikov, M.V., Perederii, M.A., Chistyakov, A.V., Konstantinov, G.I., Kadiev, K.M., and Khadzhiev, S.N., Solid Fuel Chem., 2012, vol. 46, no. 2, pp. 121–127. https://doi.org/10.3103/S0361521912020115
Tsodikov, M.V., Chistyakov, A.V., Konstantinov, G.I., Nikolaev, S.A., Borisov, R.S., Levin, I.C., Maksimov, Yu.V., and Gekhman, A.E., Russ. J. Appl. Chem., 2021, vol. 94, no. 10, pp. 1513–1524. https://doi.org/10.1134/S1070427221110069
Funding
The study was supported by the Russian Science Foundation, project no. 21-13-00457.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsodikov, M.V., Konstantinov, G.I., Chistyakov, A.V. et al. Fuel Oil Conversion in the Plasma Catalytic Mode, Stimulated by Microwave Radiation in the Presence of Nanosized Iron-Containing Systems. Pet. Chem. 62, 761–767 (2022). https://doi.org/10.1134/S0965544122050097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122050097