Abstract
The latest research results on the specific features of the process of cumene dehydrogenation to α-methylstyrene (AMS) on the porous ceramic catalytic converters were presented. The influence of the method of formation of the mono- and bimetallic rhenium- and tungsten-based components on the activity and selectivity of the synthesized converters was evaluated. It was found that the monometallic tungsten-containing converter obtained by combining self-propagating high-temperature synthesis (SHS) with the sol-gel process has the optimal composition. The experiments showed that, for this converter, the temperature range of effective operation is 550–600°C. In this range the space-time yield of AMS reached 14% at a maximum productivity of 20.57 g h–1 dm–3. The degree of carburization of the sample after 6 h of the experiment did not exceed 5 wt %, indicating its high resistance against coking.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
List of abbreviations
AMS | α-Methylstyrene |
|---|---|
SHS | Self-propagating high-temperature synthesis |
EB | Ethylbenzene |
α-Methylstyrene (AMS) is an indispensable monomer in production of styrene-butadiene rubbers and acrylonitrile-butadiene-styrene plastics possessing enhanced thermal stability and mechanical strength [1, 2]. Also, AMS is extensively used in the manufacture of adhesives, lubricating oils, perfumes, and other large-scale organic synthesis products. At present, the annual production of AMS exceeds 220 thousand tons [3]. Market research shows that, between 2017 and 2024, the average annual growth rate of the industry capacity for AMS production will be 4.5–6% worldwide. The main reason is the rise in demand for α-methylstyrene in resin manufacturing, driven by an increase in its adoption by automotive industry, which accounts for up to one-third of the AMS consumed [1].
To date, the main industrial methods for AMS production are the oxidative “cumene” process and the catalytic dehydrogenation of cumene (Eq. (1)) in adiabatic contact reactors [4]:
 (1)
(1)
The main problems with these methods are, first, insufficient purity of the resultant AMS because of an increased content of byproducts resulted from autoxidation and copolymerization with other dehydrogenate components (which calls for additional purification steps and use of multicomponent inhibitors), and, second, the need in frequent regeneration of the iron–chromium-containing catalyst used [6].
A promising route towards improving the efficiency of existing dehydrogenation processes is the development of small modular reactors based on porous ceramic catalytic converters obtained using SHS and the sol-gel process [7, 8]. This approach affords intensification of the dehydrogenation reaction and enhancement of the selectivity of the process for the target product, mainly via reducing the energy consumption for chemical conversion (compared to conventional fixed-bed reactors with granulated catalyst). This effect stems from improved heat and mass transfer in the highly porous medium of the catalytic converter. In this medium, forced diffusion of the substrate molecules in a spatially limited pore volume and advantageous ratio of the catalyst surface area to the internal pore volume are responsible for an increase in the frequency of stochastic collisions of the substrate molecules with the pore walls modified by nanosized catalytically active components, which is an important factor in catalysis. The effectiveness of this approach was confirmed by the previous studies on production of hydrogen-containing gas via reforming of organic raw materials of various origins [9, 10], as well as on preparation of valuable monomers via hydrogenation of aliphatic and aromatic hydrocarbons [11].
Another factor essential in improving the industrial technologies of basic organic synthesis is the opportunity for using catalytic converters as a replicable element of new-type modular reactors, which greatly simplifies the replacement of large volumes of catalysts and enhances the safety of production operations.
Herein, we report on the specific features of the process of cumene dehydrogenation to AMS in the catalytic channels of the porous ceramic converters modified with mono- and bimetallic rhenium- and tungsten-containing components. The choice of these components was dictated by the previously obtained experimental results, as well as by the published data on high activity and selectivity exhibited by these components in various hydrocarbon transformations [12–17].
The aim of this study was to evaluate the influence of the method of formation and composition of the catalytic converter on the process of cumene dehydrogenation to AMS.
EXPERIMENTAL
The objects of the study were porous ceramic catalytic converters modified with mono- and bimetallic rhenium- and tungsten-containing catalytic components, obtained using the SHS method and sol-gel process according to the procedures described in [12, 13].
These converters are hollow ceramic cylinders with porous gas-permeable walls, in which one end is equipped with a mounting cap for sealing the converter to the steel reactor by means of a clamping nut and other end is tightly damped to prevent gas slipping past the walls. Thus, the underlying principle of operation of the converter is forced diffusion of the reactant from the outer to the inner wall through the developed structure of tortuous catalytic channels. Figure 1 shows the external appearance of the converter.
The main parameters of the converter are as follows: total length ~115 mm; working area length (distance from the clamping nut to the damper) ~97 mm; outer diameter of the tube ~25 mm; wall thickness ~7 mm; working volume ~0.04 dm3; open pore diameter 1–3 µm; and porosity >50%.
Tables 1 and 2 list the compositions of the obtained samples. Sample no. 1, hereinafter referred to as the support, was obtained by the SHS method and mainly consists of α-Al2O3 with magnesium oxide and silicon carbide cementing additives. Modification of such supports with mono- and bimetallic rhenium–tungsten-containing catalytic coatings with the use of the sol-gel process gave sample nos. 3–6. In these sample, a γ-Al2O3 buffer layer was formed for increasing the specific surface area of the converter, and potassium and cerium oxides were deposited for decreasing the surface acidity in order to reduce the contribution from side cracking reactions accompanying the dehydrogenation of hydrocarbons and resulting in rapid coke formation on the catalyst surface [18, 19]. All the components were introduced until the limiting saturation of the pore structure of the converter with mother liquors was achieved, so their content is specific for each case and varies from sample to sample.
Sample no. 2 differs from sample no. 1 in that rhenium and tungsten were additionally introduced into the charge before sintering, whereby these catalytic components enter into the structure of the resultant converter.
The synthesized converters were tested for catalytic activity in the reaction of dehydrogenation of cumene to AMS using an original flow reactor. For details on the designs of the reactor and of the laboratory setup, as well as on the experimental procedure, see [12, 13].
Experimental Conditions
The experimental conditions were selected using the previously obtained optimal empirical data reported in the literature and the equilibrium values of the parameters of the reaction of cumene dehydrogenation to AMS [12, 13, 20–23]: substrate, cumene (98%, Sigma-Aldrich); diluent, distilled water; H2O/cumene = 14 mol/mol; flow rate: W(cumene) = 0.1 mL/min, W(H2O) = 0.2 mL/min; Т = 500–750°С; time of feedstock supply at each temperature 30 min; total time of each experiment 180 min.
Methods of Analysis of the Reaction Products
The contents of hydrogen, carbon oxides, and methane in the reaction products were determined by gas chromatography on a CRYSTALLUX–4000M chromatograph (META-CHROM, Russia) equipped with a thermal conductivity detector using high-purity argon [99.998%, GOST (State Standard) 10157-79] with a flow rate of 10 mL/min as a carrier gas. A 1 m × 3 mm adsorption column packed with SKT activated carbon with a particle size 0.2–0.3 mm was used. The column, detector, and vaporizer temperatures were 120°C. The concentrations of the gases were determined basing on the calibration curves with the use of specialized NetChrom v2.1 software.
Hydrocarbon gases С1–С5 were identified on a CRYSTALLUX–4000M chromatograph (META-CHROM, Russia) equipped with a flame ionization detector (FID) using helium [TU (Technical Specifications) 0271-001-45905715-02] as a carrier gas. The gas flow rates were as follows: helium 30 mL/min, hydrogen 35 mL/min, and air 300 mL/min. A 50 m × 0.32 mm HP-PLOT/Al2O3 (Agilent Technologies, USA) chromatographic column, film thickness 8.0 µm, was used for analysis. The column temperature was 120°C, detector temperature, 230°C, and vaporizer temperature, 250°C. The concentrations of the products were determined basing on the calibration curves with the use of specialized NetChrom v2.1 software.
Liquid organic reaction products were identified by gas chromatography-mass spectrometry (GC-MS) and gas-liquid chromatography (GLC). GC-MS analysis was performed using a Thermo Focus DSQ II instrument with a quadrupole mass analyzer (electron energy 70 eV, electron multiplier voltage 1244 V). The ion sources temperature was set at 280оС, and the interface temperature, at 280°C. The SIM (Selected Ion Monitoring) mode was used for detection.
GLC analysis was carried out on a Varian 3600 chromatograph (Varian Chromatography System, USA), equipped with a FID, a 25 m × 0.25 mm ChromTech SE-30 capillary column, Df = 0.33 μm. Oven temperature program: 50°С (5 min), 10°C/min, 280°С; injector temperature 250°С, pressure 1 bar, split ratio 1 : 200, carrier gas helium (TU 0271-001- 45905715-02).
Methods of Calculation
Conversion of cumene, wt %, was calculated using formula (2):
where mprod is the amount of the unloaded liquid reaction product, g; mcumene fed, total amount of the fed cumene, g; and Ccumene prod., concentration of cumene in the reaction products mixture, weight fraction.
The yield of styrene/AMS per fed cumene, wt %:
where Cstyrene/AMS is the concentration of styrene/AMS in the reaction products mixture, weight fraction.
The yield of styrene/AMS per converted cumene (selectivity for styrene/AMS), wt %:
where Xcumene is the conversion of cumene, weight fraction.
The content of styrene/AMS in relation to liquid byproducts, wt %:
As the main comparison criterion for evaluating the efficiency of the converters we chose the productivity for the monomer per unit working volume of the sample. This parameter is less biased than the productivity per gram of the active component, because the dimensions and gas-transport characteristics of all the obtained tubes are standardized and therefore differ insignificantly among themselves, while their masses and compositions differ markedly. The fact is that the specific features of the synthesis of the samples extremely complicate estimating the proportion of the catalytic components spent for formation of the active surface of the converter channel walls. Moreover, a challenge specific to such calculations is related to understanding the importance of each individual component (or their compositions) for the intensity of the reactions. Thus, the converter is regarded herein as an integral system combining the structural and catalytic constituents, rather than as a set of independent active species.
The productivity for styrene/AMS, g h–1 dm–3:
where Vconv is the working volume of the converter, dm3, and tfeedstock supply, feedstock supply time, min.
The increase in the productivity for styrene/AMS, relative to the support (a quantity presented for comparing the productivity on the sample of interest with that on the unmodified support (sample no. 1), taken as a standard), x times:
where ρstyrene/AMS on support is the productivity for styrene/AMS on the support, g h–1 dm–3, and ρstyrene/AMS on sample, that on the sample, g h–1 dm–3.
The procedure for calculating the presented equilibrium parameters of the reaction of cumene dehydrogenation to AMS is described in detail in [12].
RESULTS AND DISCUSSION
For the purity of the experiment, we initially carried out blank experiments on dehydrogenation of cumene to AMS in the unloaded steel reactor and in the reactor with the unmodified support mounted (sample no. 1).
The blank experiment conducted in the empty 0.2 dm3 reactor revealed low reactivity of its structural material (20Kh23N18 heat-resistant high-alloy steel). The maximum productivity for AMS, achieved at a temperature of 550°C, did not exceed 2 g h–1 dm–3 at >80 wt % cumene conversion, mostly to gaseous cracking products and to carbon.
The test results for the unmodified support (sample no. 1) showed that it in itself displayed noticeable catalytic activity in cumene dehydrogenation to AMS (Table 3). For example, at 550–600°C the productivity for AMS was 8.43–14.18 g h–1 dm–3, while the selectivity was 12.50–12.94 wt %, and the yield, 5.76–9.68 wt %. This could be attributed to the presence in the support of magnesium oxide, a cementing additive in the structure of the ceramic material, which catalyzes the proceeding chemical reactions on the sideline [12, 13, 24–26]. However, it was found thereafter that the introduction of the rhenium- and tungsten-based active components both during preparation of the support proper by the SHS method and during the subsequent formation of the catalytic film coatings on the inner surface of its pores by the sol-gel process significantly promoted the catalytic properties of the support, enhancing the selectivity and near-doubling the productivity for AMS at lower temperatures (Table 3). In view of the insignificant amounts of the catalysts used for promoting the supports (Tables 1 and 2), this finding can be considered worthy of interest.
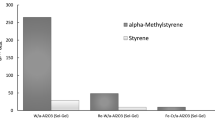
Investigation of the process of cumene dehydrogenation to AMS on a number of synthesized converters listed in Tables 1 and 2 showed that sample no. 4 modified with the tungsten-containing component represents the best converter for the process under consideration. In the range of the samples tested, this sample demonstrated the highest activity in the temperature range of 550–600°C (Table 3). According to Fig. 2, specifically in this range sample no. 4 offers the best combination of all the main output parameters of the process (cumene conversion 54.93–81.20%, AMS yield 9.82–14.04%, selectivity for AMS 17.88–17.29%, and productivity for AMS 14.38–20.57 g h–1 dm–3).
The productivity for AMS per unit working volume on the tungsten-containing converter (sample no. 4) increased relative to the support (sample no. 1) by approximately ~1.7 times at 550°C and 1.5 times at 600°C (Fig. 2). Importantly, according to the chromatography data, at 550–600°C the liquid products contained mainly unreacted cumene (75.56–41.11 wt %) and AMS (16.46–30.71 wt %) (Table 5). Thus, at 550–600°C, the content of AMS in relation to liquid byproducts in the case of the tungsten-containing converter was 67.35–52.15% at a fairly high productivity of 14.38–20.57 g h–1 dm–3. This is less characteristic of other samples and suggests high activity of the tungsten-containing converter and selectivity for the target product (Table 3).
The data on the composition of the gaseous reaction products, summarized in Table 4, indicate that, in the presence of the tungsten-containing converter (sample no. 4) at 550°C, the cumene dehydrogenation is the main process, which noticeably dominates over all the side transformations. This is evidenced by a high content of hydrogen, 96.03 vol %, at low concentrations of the cracking products, among which methane (2.10 vol %) and ethylene (1.38 vol %) are the major components. The contribution from the processes of steam reforming of hydrocarbons at this temperature also seems to be small, as demonstrated by the presence of small amounts of CO2 (0.45 vol %) resulted from steam reforming of carbon monoxide, while CO itself is clearly absent in the product gas.
At temperatures above 600°C the cumene conversion on the tungsten-containing converter (sample no. 4) exceeds the equilibrium values (Fig. 2) due to noticeable intensification of the side transformations: coking processes, cracking reactions, and steam reforming of hydrocarbons. This is indicated by a decrease in selectivity for AMS in parallel with increases in the contents of benzene and toluene in the liquid reaction products mixture (Table 5), as well as of carbon oxides and light hydrocarbons in gases (Table 4).
However, the degree of carburization of the tungsten-containing converter (sample no. 4) during the experiment (180 min) was insignificant, ≤0.13 wt % per initial converter weight (Table 6), which finding is in a good agreement with the data on dehydrogenation of various hydrocarbons, obtained in our earlier studies [11, 27, 28]. This is probably due to a higher resistance to thermal reduction in a hydrogen-saturated medium of the dehydrogenation process, displayed by tungsten(VI) oxide compared to various rhenium oxides [29, 30]. For this reason, tungsten(VI) oxide continues to be finely dispersed and does not undergo sintering on the support surface and sublimation therefrom, so that its large surface area is preserved over time. This prevents formation of carbon nuclei blocking the catalytic sites and favorably affects the efficiency of the tungsten-containing converter via increasing its operation time [11, 27, 28].
The rhenium-containing converters (sample nos. 2, 3, 5, and 6) proved to be more oriented towards cracking and reforming processes [14–17], as evidenced by increased contents of hydrocarbons and carbon oxides in the product gas throughout the temperature range examined. Moreover, these samples underwent much more pronounced coking compared to the tungsten-containing converter (sample no. 4). In the case of sample no. 3, which represents the converter modified with the rhenium monocomponent, the conversion of cumene is almost complete even at 600°C (Table 6). Furthermore, it is known that rhenium oxides are highly volatile compounds characterized by the reduction temperatures of 400–800°C [31]. Metallic rhenium particles readily fuse together, forming large clusters on the support surface [12, 13]. This causes a significant decrease in the active surface area of the catalyst and reduction in the overall efficiency of the process. All this, in our opinion, makes the rhenium-containing converter of little use for dehydrogenation processes.
A very similar result (probably for the same reasons) was demonstrated by the rhenium–tungsten-containing converter whose components were co-deposited from the solution of the bimetallic rhenium–tungsten organic complex (sample no. 6, Table 3). This may be attributed to a close proximity between the rhenium clusters formed and the finely dispersed atomic tungsten species [12, 13]. These clusters block the active tungsten sites, leading to the scenario dominated by steam reforming, typical of sample no. 3 modified with the rhenium monocomponent.
In turn, in the case of sample no. 5 containing the rhenium and tungsten components that were separately deposited on the support from independent complexes, the proceeding of cumene dehydrogenation differs greatly from that in the case of sample no. 6 containing the co-deposited components. Specifically, liquid products, including AMS, were yielded at temperatures up to 700°C (Table 5). Steam reforming is largely suppressed, as evidenced by relatively low concentrations of carbon oxides in the gas and by a much higher content of the hydrocarbons resulted from the cracking reactions (Table 4). Thus, the bicomponent rhenium–tungsten catalytic system, whose components were separately deposited, significantly alters the selectivity of the process, substantially expanding the temperature range of effective operation of the converter. The degrees of surface carburization during the experiment for separately deposited (sample no. 5) and co-deposited (sample no. 6) rhenium–tungsten-containing converters are approximately the same, averaging 4.72 wt % per converted cumene, or 0.37 wt % per initial converter weight. Nevertheless, these values exceed approximately threefold those observed for the monocomponent tungsten-containing sample no. 4 (Table 6).
The operation of the revealed mechanism of cumene dehydrogenation on the rhenium–tungsten converter, whose components were deposited separately (sample no. 5), is attributable to the size factor as applied to the catalytic particles formed on the inner surface of the pores. Apparently, the rhenium particles and tungsten particles are distant from each other when being deposited, which prevent the large rhenium clusters formed during sintering from blocking most of the finely dispersed tungsten particles. As a result, the latter remain sufficiently active, but the possible mutual influence of two different metals alters the selectivity of the process.
The rhenium–tungsten-containing converter whose active components were introduced via the SHS process (sample no. 2) deserves special attention. Table 3 shows that that this converter operates much more efficiently at elevated (600–650°C) than at moderate temperatures, which is hardly desirable for the process under study from the selectivity perspective (Table 3). Nevertheless, an exceptional advantage of the converters modified with the catalytic components in the SHS stage is reliable fixation of these components on the inner surface of the pores. Such converters withstand multiple regeneration cycles without noticeable loss of the active phase. However, a significant drawback suffered by such converters is that their preparation requires using an order of magnitude larger amount of the catalytic components compared to the surface-modified samples (Tables 1 and 2). The fact is that, due to the specific features of proceeding of the SHS process, the main part of these components is embedded deep into the structure of the ceramic material and thus is inaccessible to the reactants, rather than occurs on the surface which is open for the substrate molecules. This lowers the economic efficiency of the process involving the use of a rare and an expensive metal such as rhenium.
CONCLUSIONS
Our studies showed that the modification of the porous ceramic tubular support by the rhenium- and tungsten-based active components significantly promotes its catalytic properties, enhancing the selectivity and near-doubling the productivity for AMS at lower temperatures.
It was demonstrated that the monocomponent tungsten-containing converter (sample no. 4) obtained using the SHS and the sol-gel processes is the most effective for AMS production by cumene dehydrogenation, providing high yield, selectivity, and productivity for the target product over a moderate temperature range of 550–600°С. Moreover, this converter is the most coke-resistant among the samples tested. This is attributable to the finely dispersed distribution of the tungsten particles that do not undergo sintering on the support surface and prevent carbon nucleation.
The rhenium-containing converters (sample nos. 2, 3, 5, and 6) are more oriented towards cracking, reforming, and coking processes, which noticeably reduces their efficiency in the AMS production process.
The rhenium–tungsten-containing converter modified by the catalytic components in the stage of self-propagating high-temperature synthesis (sample no. 2) has the advantage of providing reliable fixation of these components on the inner surface of the pores. This converter withstands multiple regeneration cycles without noticeable loss of the active phase. However, its major drawback is that its preparation requires an order of magnitude larger amount of the catalytic components compared to the surface-modified samples. The reason is that, owing to the specific features of proceeding of the SHS process, the main part of these components is embedded deep into the structure of the ceramic material and thus is inaccessible to the reactants, rather than occurs on the surface which is open for the substrate molecules. This lowers the economic efficiency of the process involving the use of a rare and an expensive metal such as rhenium.
Change history
27 August 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S0965544122070192
REFERENCES
https://www.gminsights.com/industry-analysis/alpha-methyl-styrene-market
https://www.persistencemarketresearch.com/market-research/alpha-methyl-styrene-market.asp
https://www.icis.com/explore/resources/news/2000/05/22/115657/alpha-methylstyrene/
Litvin, O.B., Osnovy tekhnologii sinteza kauchukov (Fundamentals of Rubber Synthesis Technology), 2nd ed., Moscow: Khimiya, 1964.
Sinteticheskii kauchuk (Synthetic Rubber), Garmonov, I.V., Ed., Leningrad: Khimiya, 1976.
Lavrenov, A.V., Saifulina, L.F., Buluchevskii, E.A., and Bogdanets, E.N., Catal. Ind., 2015, vol. 7, no. 3, pp. 175–187. https://doi.org/10.1134/S2070050415030083
Kurchatov, I.M., Laguntsov, N.I., Tsodikov, M.V., Fedotov, A.S., and Moiseev, I.I., Kinet. Catal., 2008, vol. 49, no. 1, pp. 121–126. https://doi.org/10.1134/S0023158408010151
Tsodikov, M.V., Fedotov, A.S., Antonov, D.O., Uvarov, V.I., Bychkov, V.Y., and Luck, F.C., Int. J. Hydrogen Energy, 2016, vol. 41, no. 4, pp. 2424–2431. https://doi.org/10.1016/j.ijhydene.2015.11.113
Fedotov, A.S., Antonov, D.O., Bukhtenko, O.V., Uvarov, V.I., Kriventsov, V.V., and Tsodikov, M.V., Int. J. Hydrogen Energy, 2017, vol. 42, no. 38, pp. 24131–24141. https://doi.org/10.1016/j.ijhydene.2017.07.095
Fedotov, A.S., Antonov, D.O., Uvarov, V.I., Tsodikov, M.V., Paul, S., Heyte, S., and Dumeignil, F., Petrol. Chem., 2019, vol. 59, no. 4, pp. 405–411. https://doi.org/10.1134/S0965544119040066
Fedotov, A.S., Uvarov, V.I., Tsodikov, M.V., Paul, S., Simon, P., Marinova, M., and Dumeignil, F., Petrol. Chem., 2020, vol. 60, no. 11, pp. 1268–1283. https://doi.org/10.1134/S0965544120110080]
Fedotov, А.S., Uvarov, V.I., Tsodikov, M.V., Paul, S., Simon, P., Marinova, M., and Dumeignil, F., Chem. Eng. Proc., Proc. Intens., 2021, vol. 160, p. 108265. https://doi.org/10.1016/j.cep.2020.108265
Ryashentseva, M.A. and Minachev, Kh.M., Usp. Khim., 1969, vol. 38, no. 11, pp. 2050–2074.
Ryashentseva, M.A. and Minachev, Kh.M., Renii i ego soedineniya v geterogennom katalize (Rhenium and Its Compounds in Heterogeneous Catalysis), Moscow: Nauka. 1983.
Ryashentseva, M.A., Usp. Khim., 1998, vol. 67, no. 2, pp. 175–196.
Ryashentseva, M.A., Vestn. Mosk. Inst. Tonk. Khim. Tekhnol., 2007, vol. 2, no. 2, pp. 12–26.
Lebedev, N.N., Khimiya i tekhnologiya osnovnogo organicheskogo i neftekhimicheskogo sinteza: Uchebnoe posobiye (Chemistry and Technology of Basic Organic and Petrochemical Synthesis: Textbook), Moscow: Khimiya, 1988.
Wang, H. and Tsilomelekis, G., Catal. Sci. Technol., 2020, vol. 10, no. 13, pp. 4362–4372. https://doi.org/10.1039/D0CY00586J
Green, D.W. and Perry, R.H., Perry’s Chemical Engineers’ Handbook, New York: McGraw-Hill, 8th ed., 2008.
Bashkatov, T.V. and Zhigalin, Ya.L., Tekhnologiya sinteticheskikh kauchukov (Synthetic Rubber Technology), Leningrad: Khimiya, 1987.
Adel’son, S.V., Vishnyakova, T.P., and Paushkin, Ya.M., Tekhnologiya neftekhimicheskogo sinteza: Uchebnik dlya vuzov (Technology of Petrochemical Synthesis: Textbook for Higher Education), Moscow: Khimiya, 1985.
Thomas, C.L., Catalytic Processes and Proven Catalysts, New York: Academic, 1970.
Ismailov, R.G., Promyshlennaya pererabotka nefti i razvitie neftekhimii (Industrial Oil Refining and Development of Petrochemistry), Baku: Azerbaidzhan. Gos. Izd., 1964.
Vaculík, P., Chemie Monomerů, Praha: Československá akademie věd, 1956, Vol. 1.
Uvarov, V.I., Loryan, V.E., Borovinskaya, I.P., Shustov, V.S., Fedotov, A.S., Antonov, D.O., and Tsodikov, M.V., Refract. Ind. Ceram., 2018, vol. 59, no. 2, pp. 215–217. https://doi.org/10.1007/s11148-018-0208-2
Fedotov, A., Konstantinov, G., Uvarov, V., Tsodikov, M., Paul, S., Heyte, S., Simon, P., and Dumeignil, F., Catal. Commun., 2019, vol. 128, p. 105714. https://doi.org/10.1016/j.catcom.2019.105714
Fedotov, A.S., Uvarov, V.I., Tsodikov, M.V., Moiseev, I.I., Paul, S., Heyte, S., Simon, P., and Dumeignil, F., Kinet. Catal., 2020, vol. 61, no. 3, pp. 390–404. https://doi.org/10.1134/S002315842003009X
Romanyuk, A., Steiner, R., Oelhafen, P., Biskupek, J., Kaiser, U., Mathys, D., and Spassov, V., J. Phys. Chem., 2008, vol. 112, no. 30, pp. 11090–11092. https://doi.org/10.1021/jp803844d
Wilken, T.R., Morcom, W.R., Wert, C.A., and Woodhouse, J.B., Metall. Transact. B, 1976, vol. 7, no. 4, pp. 589–597.
Lai, C., Wang, J., Zhou, F., Liu, W., and Miao, N., J. Alloys Compd., 2018, vol. 735, pp. 2685–2693. https://doi.org/10.1016/j.jallcom.2017.11.064
Funding
This study was supported by the Russian Science Foundation (project no. 17-13-01270-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
M.V. Tsodikov, a co-author, is an Editorial Board member at the Neftekhimiya (Petroleum Chemistry) Journal. The other co-authors declare no conflict of interest requiring disclosure in this article.
Additional information
Translated from Neftekhimiya, 2022, Vol. 62, No. 4, pp. 548–560 https://doi.org/10.31857/S0028242122040104.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fedotov, A.S., Bagdatov, R.A., Grachev, D.Y. et al. Composition and Preparation Method of Rhenium- and Tungsten-Containing Porous Ceramic Converters Influence on the Cumene Dehydrogenation to α-Methylstyrene Process Specific Features. Pet. Chem. 62, 660–671 (2022). https://doi.org/10.1134/S0965544122040090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122040090