Abstract
The study focuses on corrosion challenges for naphtha hydrotreating equipment (installed upstream of a reforming unit) caused by ammonium chloride deposits and on the development of adequate chemical engineering protection measures. Using an experimental setup, we investigated the sublimation/desublimation of ammonium chloride at varying pressures and temperatures in a medium typical of naphtha and hydrogen-bearing gas streams flowing from a naphtha hydrotreater. Superstoichiometric concentrations of hydrogen chloride were found to significantly decrease the desublimation temperature (by 30–50°C) compared to a system that has a stoichiometric HCl concentration. Consequently, solid-phase ammonium chloride exists in a wider range of temperatures and pressures. Introducing surfactants (especially higher alcohols) into the system reduces adhesion and affects the crystalline structure of salt deposits—despite the solid phase being stabilized by an excess (superstoichiometric) concentration of hydrogen chloride—which makes surfactants suitable for mitigating the salt deposition challenge. Based on the data obtained, we proposed effective chemical engineering protection methods that use water-soluble corrosion inhibitors in combination with an online automated system for organochlorine control.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Russian Federation, as well as some other oil-producing countries, periodically faces the challenge of the entry of organochlorines into pipeline systems and refineries. For example, the organochlorine ingress to the Druzhba main pipeline in 2019 led to major issues for the transportation and refining of the affected crude oil.
A common challenge in naphtha hydrotreating is the concomitant hydrogenolysis of organochlorine and organonitrogen compounds to hydrogen chloride and ammonia [1], which results in equipment corrosion and ammonium chloride deposits. There has been very limited publicly available information on the physicochemical behavior of the hydrogenolysis products, and of ammonium chloride produced from their mutual reaction, under hydrotreating and reforming process conditions [2]. Even though a similar problem has been explored in a prior study [3], its findings require further elaboration under industrial hydrotreating operating conditions.
Hydrogen sulfide, hydrogen chloride, and water cause vigorous destruction of steels [4]. The combined synergistic effect of these compounds is responsible for a high corrosion rate in equipment and piping exposed to depressurizing, such as air coolers and pipeline sections downstream of constrictions (e.g., membranes and valves). To control this type of corrosion, the most effective approach is to implement an integrated chemical engineering protection system, primarily based on corrosion inhibitors. An individual condition-specific equipment protection program must be developed for each process unit [5]. Details of this chemical engineering protection and specific dosage of reagents must be determined by each refinery based on the process characteristics and pilot test data of the process unit.
The typical chemical engineering protection for hydrotreating equipment consists of the following measures [6–8]:
(1) Continuous introduction of a water-soluble corrosion inhibitor (in the form of a steam condensate solution) into stripper overhead lines. The water-soluble form of an inhibitor facilitates its subsequent removal along with wash water and minimizes the entry of its nitrogenous components to the reformer catalyst unit (where these components may otherwise deactivate the catalyst acid sites). The reagent should be injected to the overhead line upstream of the air coolers, the specific injection point being determined directly in the field based on the location of the pipelines and service platforms. It is recommended that the inhibitor injection points be installed in the horizontal line section at least six line diameters downstream from a configuration break point (e.g., branch, reducer, bend, etc.). Injecting the inhibitor as a steam condensate solution through spray nozzles maximizes the desired effect; and
(2) Corrosion protection monitoring. This should include both direct and indirect methods for assessing current corrosion conditions.
Direct methods are: installation of corrosion probes and corrosion rate evaluation using corrosion coupons; and intermittent wall thickness gauging.
Indirect methods include: regular testing of water from stripper reflux drums to evaluate its quality parameters such as pH and the concentrations of dissolved iron, ammonium ions, and chlorine ions.
In a reforming system, ammonium chloride is permanently present in the hydrotreating process streams. The thermal behavior of pure ammonium chloride has been well studied [9]. Prior research has demonstrated [3] that, at temperatures below 340°C and at a pressure of 1.5 to 3.0 MPa (gauge), NH4Cl particles become stable and present in the gas/vapor phase as fine aerosol. However, under industrial conditions, superstoichiometric concentrations of HCl add a major challenge to the utilization of NH4Cl deposition inhibitors.
The purpose of the present study was to investigate the phase equilibrium of systems that contain ammonium chloride, hydrogen chloride, water, and hydrocarbons, under real-world naphtha hydrotreating conditions. Our goal was to configure, based on this investigation, the optimal chemical engineering protection.
EXPERIMENTAL
Using a customized experimental setup (Fig. 1), we tested the sublimation/desublimation of ammonium chloride at varying pressures and temperatures. The test was carried out in a medium typical of naphtha and hydrogen-bearing gas streams flowing from a naphtha hydrotreater. Sublimation was examined in H2-bearing gas flow (81 vol % H2, the balance consisting of methane, ethane, and other hydrocarbons). The experimental design enabled us either to saturate the feed gas with water vapor or to dehydrate the feed gas using zeolites. The water-filled separator was equipped with a jacket connected to an oven to provide a specific temperature in the vessel. Furthermore, the design allowed us to vary the gas humidity from 2 to 300 ppm. Zeolite NaX (TU 2163-077-05766575-99, rev. 1–7) was utilized for drying.
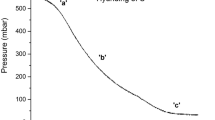
The tubular reaction column (reactor), functionally similar to a heat exchanger, was equipped with removable plates (Fig. 2) to measure ammonium chloride sublimation. Across the column length, we induced a temperature gradient, which was monitored by 11 thermocouples spaced lengthwise. A weighed NH4Cl sample was placed on the lower trays. A carrier gas with set humidity was then fed to the column bottom. The system was held until equilibrium was reached, after which the lengthwise temperature profile was recorded (Fig. 3). The sublimated ammonium chloride was collected and weighed. The initial and final sublimation/desublimation temperatures were derived from the distribution of NH4Cl crystals. The crystal morphology was examined by optical microscopy (using an MBS-10 instrument).
We further investigated the surfactant effects on the crystallization of the sublimated ammonium chloride, particularly on its physicochemical properties. For this purpose, pre-calculated amounts of surfactants were added to the salt sample. Organonitrogen and organooxygen compounds differing in type and composition were used as the surfactants:
—Commercial nitrogen-containing inhibitor 1;
—Commercial nitrogen-containing inhibitor 2;
—Higher alcohols (C6–C18 distillate);
—Butanol bottoms (from the Angarsk Petrochemical Company, Russia);
—Phenol (TU 6-09-40-3245-90; manufactured by LenReactiv, Russia);
—4-Methyl-2,6-di-tert-butylphenol (technical grade according to TU 38.5901237-90, rev. 4–9; manufactured by the Sterlitamak Petrochemical Plant, Russia); and
—1,2-Dihydroxybenzene (≥99%; manufactured by Sigma-Aldrich).
To quantify the effects of various surfactants on ammonium chloride desublimation, a number of GOST St3 steel treated-surface plates (ethanol-washed and sanded with GOST 3647-80 M28/N-2 paper) were installed in the desublimation zone. The plates were numerated and weighed. Each plate was placed in the desublimation zone of the experimental reactor. Following the test run, the plates were weighed before and after removing (by single shaking) the ammonium chloride. The ratio of the desublimated NH4Cl weight loss to the NH4Cl weight after the plate shaking was evaluated in percent. This ratio characterized the surfactant effect on the strength of adhesion of NH4Cl crystals to the plate surface.
RESULTS AND DISCUSSION
The study began with investigating the initial temperatures of NH4Cl sublimation and decomposition as pressure functions. These data enabled us to present the phase of NH4Cl as a function of temperature and pressure in a hydrogen medium under superstoichiometric HCl conditions (Fig. 4).
The initial sublimation temperature was found to increase with process pressure. Therefore, a decrease in the heat exchanger temperature and pressure (caused by hydrocarbon condensation in the hydrotreater outlet streams that contain ammonium chloride vapor) necessarily leads to the desublimation and recovery of the NH4Cl in a solid phase. Moreover, the Venturi depressurizing effect, which results from the fluid flowing through a constricted (due to NH4Cl clogging) diameter of the heat exchanger tubes, accelerates the subsequent desublimation of ammonium chloride, thus creating a positive feedback effect.
To identify the water vapor contribution to the NH4Cl sublimation/desublimation, we conducted a series of experiments with varying reactor humidity. The lowest moisture content was 2 ppm. These conditions were referred to as a dry system. For the wet system case—which corresponds to an equilibrium moisture content in a hydrocarbon feedstock when in contact with condensed water under nominally normal conditions of process pipelines and vessels—the humidity was assumed to exceed 100 ppm.
The morphology investigation indicated that, in the dry sublimation/desublimation system, the resultant NH4Cl crystals were larger in size and tended to be concentrated in large clusters on the tray surfaces. In the wet system case, fine NH4Cl crystals were dissipated in a thin layer, with a 15–30% larger coverage area than that in the dry system.
Based on the measurement statistics, we further plotted the NH4Cl sublimation process pattern as a temperature function for three different pressure levels typical of naphtha hydrotreating with stoichiometric and superstoichiometric NH4Cl concentrations (Fig. 5). Introducing water vapor into the system was found to lower the NH4Cl sublimation temperature within the entire pressure range under study [3]. This effect is likely associated with the ionic action of water dipoles on ammonium chloride molecules and with hydrolysis:
Figure 6 presents the ammonium chloride desublimation temperature as a function of the reaction column humidity at 2 MPa. The plot clearly shows that humidity above 30–40 ppm leads to a major decrease in the sublimation temperature. This is very important for the process design of hydrotreating equipment. The following empirical equation for the relationship between the desublimation temperature and the gas humidity was derived from the data statistics:
where Td is the desublimation temperature (°C), and H is the gas humidity (ppm).
This empirical equation explains the experimental data with an R-square of 0.9919, thus indicating good approximation.
To confirm the controllability of NH4Cl desublimation, the surfactant effects on the crystallization of the sublimated NH4Cl and its physicochemical properties were examined. It was shown that the sublimation of ammonium chloride in the presence of surfactants affects the crystal shape, the crystallization zone, and the adhesion of the crystals to the steel and to one another.
Table 1 presents data on ammonium chloride weight loss under the influence of various surfactants. In all the experiments, a surfactant concentration of 30 ppm per unit weight of NH4Cl was used. Although higher or lower reagent concentrations could also be used, it was particularly important to optimize the reagent dosage for industrial hydrotreating operating conditions.
A dispersing effect was revealed for some surfactants: they are able to alter the adhesion of ammonium chloride crystals, both between themselves and to the steel surface. The study data demonstrate that the presence of NH4Cl affected the phase equilibriums of the mixture components and, even more importantly, those of water. A previous study that focused on systems that contained both water and ammonium chloride showed a 15°C drop in the water dew point [3]. On the other hand, additional amounts of hydrogen chloride and hydrogen sulfide have almost no effect on the water dew point decline. Furthermore, the low surfactant concentrations (not exceeding a few dozen ppm) used in our study will not harm reforming catalysts (especially in the case of nitrogen-free surfactants) when being utilized in a real-world naphtha hydrotreating unit.
The above-mentioned effect is known to promote water condensation in heat-exchange equipment to form a separate aqueous layer saturated with hydrogen sulfide, hydrogen chloride, ammonia, and related salts. This aqueous layer generally exhibits pH < 3 and is highly corrosive. In contrast, dry HCl has no noticeable corrosive effect on carbon steels and alloy steels [10] due to the lack of an electrolytic environment. We observed a similar case for ammonium chloride: in the presence of water, this compound hydrolyzed and caused vigorous undersludge pitting corrosion of steels.
CONCLUSIONS
In the course of the study, we developed a methodology for the investigation of ammonium chloride sublimation/desublimation under the simulated operating conditions of industrial naphtha hydrotreating equipment installed upstream of a reforming unit. The effects of process conditions, such as pressure and temperature, on the NH4Cl sublimation/desublimation parameters were elaborated. In particular, superstoichiometric concentrations of hydrogen chloride significantly decrease the desublimation temperature compared to a system that has a stoichiometric HCl concentration. Consequently, solid-phase ammonium chloride exists in a wider range of temperatures and pressures. These effects, in turn, hinder the dispersion of ammonium chloride, including in those cases where deposition inhibitors are used. Introducing surfactants into the system reduces adhesion and affects the crystalline structure of salt deposits—despite the solid phase being stabilized by an excess (superstoichiometric) concentration of hydrogen chloride—which makes surfactants suitable for mitigating the salt deposition challenge. The implementation of the proposed chemical engineering protection methods, in combination with an online automated system for organochlorine control, offers prospects for refining organochlorine-rich crude oils without the need to significantly modernize the process design.
REFERENCES
Treese, S., Digital Refining, October, 2019. https://www.digitalrefining.com/article/1002443/the-origins-and-fates-of-chlorides-in-hydroprocessing-units#.YjRWRVVByUk
Erfan, M., Digital Refining, March, 2011. https://www.digitalrefining.com/article/1000407/chloride-removal-in-refineries#.YjRWdlVByUk
Tomin, V.P. and Kabyshev, V.A., Neftepererab. Neftekhim. Nauch.-Tekhn. Dostizh. Pered. Opyt, 2009, no. 7, pp. 11–15.
Uglig, H.H. and Revie, R.W., Corrosion and Corrosion Control. An Introduction to Corrosion Science and Engineering, University of Michigan, 1971.
Aslam, R., Mobin, M., and Aslam, J., Environmentally Sustainable Corrosion Inhibitors, 2022, vol. 19, pp. 385–404. https://doi.org/10.1016/B978-0-323-85405-4.00004-5
Murashchenko, M.G., Kamalov, K.G., Khutoryanskii, F.M., Tsvetkov, A.L., Andzhaev, S.S., and Voronina, N.A., Mir Nefteprod. Vestn. Neft. Komp., 2012, no. 11, pp. 15–17.
Tomin, V.P. and Kabyshev, V.A., Neftepererab. Neftekhim. Nauch.-Tekhn. Dostizh. Pered. Opyt, 2009, no. 1, pp. 45–48.
Kolotov, V.Yu., Tomin, V.P., Kolyvanova, E.M., and Krashchuk, S.G., Neftepererab. Neftekhim., 2003, no. 8, pp. 36–40.
Zaitsev, P.M., Tavrovskaya, A.Ya., Podlesskaya, A.V., and Portnova, N.L., Trudy NIUIF, Moscow, 1982, pp. 154–168.
Semenova, I.V., Florianovich, G.M., and Khoroshilovo, A.V., Korroziya i zashchita ot korrozii (Corrosion and Corrosion Protection), Semenova, I.V., Ed., Moscow: Fizmatlit, 2002.
Funding
The study was financed by TIPS RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Translated from Neftekhimiya, 2022, Vol. 62, No. 2, pp. 209–215 https://doi.org/10.31857/S0028242122020034.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Korenev, V.V., Tomin, V.P., Zhdaneev, O.V. et al. Phase Equilibriums of Ammonium Chloride Systems as Model Hydrogenolysis Products of Organochlorine Compounds under Naphtha Hydrotreating Conditions. Pet. Chem. 62, 376–382 (2022). https://doi.org/10.1134/S0965544122020177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122020177