Abstract
Thermal cracking of C5-deasphalted oil (C5-DAO) was performed in a batch reactor at 410°C in the absence and presence of H2 gas and catalysts. Coke was formed during the thermal cracking of C5-DAO even though the feed contained no asphaltenes. Although H2 suppressed asphaltene formation and decreased the coke yield, coke was still formed in the absence of a catalyst. A slurry-phase dispersed catalyst inhibited the transformation of resins into heavier fractions and facilitated <1 wt % asphaltene yield with no coke formation even under high conversion conditions. The main function of the catalyst was the facilitation of hydrogenation reactions and the stabilization of free radicals, leading to liquid production by inhibiting coke formation. Consequently, the product quality, in terms of hydrogen to carbon ratio and microcarbon residue and S contents, was enhanced by catalytic hydrocracking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Research and development of heavy oil upgrading technologies has recently attracted considerable attention because the demand for high quality fuel oils has increased while the proportion of low quality crude oil has grown [1–5]. Among heavy oil feedstocks, vacuum residue (VR), the product from the bottom of the vacuum distillation column in the crude oil refining process, contains relatively high asphaltene, S, N, and metal (Ni and V) contents [6–7]. Asphaltenes, the heaviest and most polar fraction of heavy oils, limit the heavy oil upgrading process because of the formation of deposits leading to fouling and catalyst deactivation [8–14], thus negatively affecting the process economics [15].
It has been reported that the quality of feedstock decreases with increasing asphaltene content [7]. Thus, the coke yield increases significantly, and the liquid product yield decreases when feeds with high asphaltene content are used for hydrocracking reactions. Moreover, feeds with low asphaltene content showed higher hydrocracking ability (increase in American Petroleum Institute gravity) and impurity (S and metal) removal than feeds with high asphaltene content [16]. In addition, isolated asphaltenes were upgraded directly by thermal treatment to elucidate the behavior of asphaltene molecules [17–21]. Asphaltenes were easily converted to coke due to the dealkylation of aliphatic chains through thermal treatment, resulting in hydrogen depletion and carbon enrichment. Therefore, the removal of asphaltenes from heavy oil feedstocks is an effective solution for reducing problems associated with the heavy oil upgrading process as well as for enhancing the quality of liquid products.
The solvent deasphalting (SDA) process facilitates the recovery of deasphalted oil (DAO) from VR by removing asphaltenes and heavy resins (which are insoluble in deasphalting solvents), resulting in the removal of metals and S from feedstocks with asphaltenes. It has been reported that DAO hydrocracking is typically performed using heterogeneous catalysts such as Co–Mo–Ni/Al2O3 [16, 22–24]. DAO showed higher hydrocracking activity than VR, but coke was still formed from the resins and aromatic feedstock during the DAO hydrocracking in the presence of a NiMo/Al2O3 catalyst [23]. Moreover, the products obtained from DAO hydrocracking in the presence of NiMoS/Al2O3 and NiMoS–TiO2–USY catalysts were distilled into five fractions (gasoline, kerosene, gas oil, vacuum gas oil, and resins) and characterized by gas chromatography-atomic emission detection (GC-AED) to evaluate the hydrocracking performance by determining the distribution of C, S, and N species in the products [24].
Previous studies have evaluated the performance of DAO hydrocracking on the basis of the change in distilled fractions in liquid products; however, this change does not indicate the change in the chemical structure of the products or the role of catalysts and H2. In addition, although hydrocracking of DAO was easier than that of VR due to asphaltene removal, coke was still formed during DAO hydrocracking over the commercial heterogeneous catalysts, wherein the catalyst was deactivated. Therefore, a slurry-phase dispersed Mo-octoate precursor was used in this study to investigate whether coke formation could be controlled by the dispersed catalyst when the feed did not contain asphaltenes.
In this study, thermal cracking and non-catalytic hydrocracking of C5-DAO were performed to elucidate the effect of H2 in comparison with N2. The aggregation tendency of resins in DAO after non-catalytic hydrocracking was investigated by X-ray diffraction (XRD) of asphaltenes and coke. The catalytic hydrocracking of C5-DAO was also conducted in a semi-batch system to determine the role of the Mo-octoate catalyst precursor; catalytic hydrocracking was compared to non-catalytic hydrocracking in terms of hydrocracking activity, product quality, and saturates, aromatics, resins, and asphaltenes (SARA) distribution. The SARA distribution indicated the change in the chemical structure of the products obtained from non-catalytic and catalytic hydrocracking, which facilitates deep understanding of the role of the catalyst.
EXPERIMENTAL
Separation of C5-DAO feedstock and products. C5-DAO was separated from VR that was obtained from the Hyundai Oilbank Corporation (Korea) using a solvent extraction method with n-pentane, and it was used as a feedstock in this study. The scheme of the separation method is shown in Fig. 1. The procedures for the separation of C5-DAO were the same as the methods mentioned in our previous study [6]. The properties of C5-DAO and VR were listed in Table 1. The VR exhibited a high asphaltene concentration of 25.0 wt %. A solvent extraction method removed asphaltenes from the VR to obtain an asphaltene-free C5-DAO feedstock. This indicates that the solvent extraction method effectively leads to a reduction in the amount of microcarbon residue (MCR), N, S, and metals.
Cracking experiments. Thermal cracking and non-catalytic hydrocracking of C5-DAO were performed in a 100 mL Parr reactor with a batch system in the presence of N2 and H2 gases, respectively. A total of 20 g of C5-DAO was added into the reactor, and the gas was supplied at 80 bar at 80°C. The temperature was increased from 80°C to the desired reaction temperature of 410°C. The reactions were carried out for 0.5–12 h under vigorous stirring (1500 rpm). The detailed reaction procedure was similar to the method mentioned in our previous study [7].
The catalytic hydrocracking of C5-DAO was conducted in a semi-batch system with 110 bar of H2 and 1000 ppm of Mo from a Mo-octoate catalyst precursor (15.4 wt % Mo purchased from Shepherd) at 410°C. In the batch system, reaction pressure was not constant and decreased because of H2 consumption or increased because of gas formation. In contrast, in the semi-batch system, the reaction pressure was maintained by the continuous addition of H2 through a mass flow controller (MFC). The reaction pressure was fixed at 110 bar by a TESCOM regulator. The volume of H2 added during the reaction was recorded to calculate the H2 consumption.
Characterization. The catalyst dispersed in the liquid product was adsorbed and concentrated on the activated carbon for accurate analysis. After the catalytic hydrocracking of C5-DAO, the mixture of liquid products (including Mo species) and activated carbon (Sigma-Aldrich) was added to toluene, then it was stirred at 300°C under an N2 atmosphere for 1 h in the closed system. The solid was separated from the liquid by vacuum filtration, and it was dried at 110°C overnight. The activated carbon with the adsorbed catalysts were examined by transmission electron microscopy (TEM; Talos F200S microscope), and X-ray photoelectron spectroscopy (XPS; Kratos AXIS NOVA spectrometer with AlKα radiation) was conducted to determine the morphology of the Mo active species after the hydrocracking reactions. The XPS spectra of Mo 3d were recorded, and the binding energy (BE) was referred to as the energy of the C 1s peak (BE = 284.8 eV).
The light hydrocarbons and remaining H2 after the reaction were analyzed by gas chromatography (GC) with a thermal conductivity detector. The MCR content was measured by a MCR tester (MCRT-160) following ASTM D 4530-03. The saturate, aromatic, resin, and asphaltene contents of DAO product were identified by a SARA instrument (IATROSCAN MK-6s) with a Chromarod-S5 column. Simulated distillation (SIMDIS) GC was carried out using Agilent GC 7890 according to ASTM 7169 to determine the distillation curves of the liquid products. Elemental analysis (EA; Thermo Scientific Flash EA-2000 Organic Elemental Analyzer) and inductively coupled plasma-atomic emission spectrometry (ICP-AES; Thermo Scientific iCAP 7400 Duo) were conducted to determine the contents of C, H, N, and S as well as Ni and V in the samples. Carbon-13 nuclear magnetic resonance analysis was conducted on a Bruker Avance 500 spectrometer. The DAO product was dissolved in a CDCl3 solvent (99.8%, Sigma-Aldrich without TMS) including 0.1 M chromium(III) acetylacetonate [Cr(acac)3, Sigma-Aldrich] as a relaxation agent [25].
XRD measurements of the asphaltenes and coke formed through the reaction were performed in a high power Rigaku Ultima IV Diffractometer. The diffraction patterns (2θ = 6°–80°) were recorded at room temperature using CuKα radiation (λ = 1.5418 Å) of 40 kV and 40 mA at a scanning speed of 1 min–1 and a step size of 0.02° (2θ). The XRD patterns were smoothed and deconvoluted in the region of 2θ = 8°–35° by Peak Fit software version 4.12 from Systat Software, Inc (USA). The macrostructure parameters of asphaltenes and coke, such as aromaticity (fa-XRD), distance between the neighboring aromatic sheets (dm), average height of the stacked aromatic sheets perpendicular to the plane of the sheet (Lc), and number of aromatic sheets in a stacked cluster (M), were calculated using Eqs. (1)–(4), which have been mentioned in previous studies [6, 26–28].
RESULTS AND DISCUSSION
Comparative activity between thermal cracking and non-catalytic hydrocracking of C5-DAO. Series experiments on thermal cracking and the non-catalytic hydrocracking of C5-DAO as a function of reaction time were performed to elucidate the effect of H2 on the thermal cracking of C5-DAO. The liquid was the most important and valuable product in the oil upgrading process, which decreased as the reaction went on due to conversion into coke and gas phases. To investigate the upgrading degree, liquid products were classified into four components with different distillation intervals, such as naphtha (IBP–177°C), middle distillate (177–343°C), vacuum gas oil (343–524°C), and residue (524°C–FBP).
Table 2 summaries the liquid product yield, residue conversion, H2 consumption, SIMDIS, and SARA fractions of the liquid products obtained from the thermal cracking, non-catalytic hydrocracking, and catalytic hydrocracking of C5-DAO as a function of reaction time. The residue conversion obtained from the thermal cracking were similar to that obtained from the non-catalytic hydrocracking, whereas the total yield for liquid products of the thermal cracking was slightly lower than that of the non-catalytic hydrocracking at the same reaction time. This is because more residue was converted into coke during the thermal cracking than the non-catalytic hydrocracking as shown in Fig. 2. The residue conversion increased steadily as the reaction time increased. It was also observed that light fractions such as the naphtha and middle distillate increased while the vacuum gas oil decreased with increased residue conversion. This indicates that the cracking of heavy oils into light fractions is driven by thermal energy. Figure 2 shows the distribution of gas, DAO product, asphaltenes, and coke yields obtained from the thermal cracking, non-catalytic hydrocracking, and catalytic hydrocracking of C5-DAO. It was observed that coke was formed during the thermal cracking of C5-DAO even though the feed contained no asphaltenes. This indicates that the coke could be formed not only from asphaltenes but also from resins that are also heavy compounds like asphaltenes. The thermal cracking showed a higher yield of coke than the non-catalytic hydrocracking reaction, as shown in Fig. 2. The yield of asphaltenes increased rapidly at the initial reaction time during both the thermal cracking and non-catalytic hydrocracking and then slightly decreased due to the formation of coke from the asphaltenes. For thermal cracking, asphaltene yield increased more sharply than that for the non-catalytic hydrocracking, and it decreased at 2 h. It is well known that slurry phase hydrocracking proceeds through thermolysis driven by free radicals [29, 30], which promotes the condensation of polyaromatic compounds as well as the β-scission of alkyl substituents. In catalytic hydrocracking, the corner and edge sites occupied by S ions in MoS2 can easily be interchanged with H2 to generate active hydrogen, resulting in the suppression of radical polymerization [31, 32]. It has been also reported that hydrocarbon free radicals were terminated by H2 molecules [30]. Although the hydrogen transfer rate of non-catalytic hydrocracking was slower than that of catalytic hydrocracking, non-catalytic hydrocracking showed slightly better reaction results with a lower coke yield than the thermal cracking reaction because H2 led the occurrence of the hydrogenation reaction and stabilized the free radicals during the thermal cracking reaction. It was also observed that H2 consumption per 1 g feedstock increased as the reaction time increased from 1 to 8 h (Table 2).
Gas, DAO-product, asphaltenes, and coke yields obtained from (1) thermal cracking, (2) non-catalytic hydrocracking, and (3) catalytic hydrocracking of C5-DAO feedstock. Reaction conditions: T = 410°C, P = 80 bar at 80°C for thermal cracking and non-catalytic hydrocracking and 110 bar with 1000 wppm of Mo from Mo-octoate for catalytic hydrocracking.
Aggregation of resins in C5-DAO during non-catalytic hydrocracking. It has been reported that the solubility of asphaltenes decreased during the hydrocracking process because the aromaticity of the asphaltenes increased via cracking the aliphatic chains of the asphaltene molecules, which resulted in the aggregation and precipitation of asphaltenes into coke [6, 15, 33–35]. Resins have smaller and fewer polar molecules than asphaltenes, but the molecular structures are similar [36]. Therefore, it was supposed that the formation of asphaltenes and coke from C5-DAO could be attributed to the aggregation of resins in C5-DAO feedstock during the non-catalytic hydrocracking reactions. After the reactions, DAO product was separated from the total liquid product by removing asphaltenes and coke. The change of SARA fractions in DAO product obtained from the non-catalytic hydrocracking of C5-DAO feedstock was also listed in Table 2. During the reactions, the saturates slightly decreased due to the formation of gas products via cracking reactions, while aromatics became almost constant. It was also observed that resins decreased as a result of cracking into light fractions as well as aggregation that formed asphaltenes, as shown in Fig. 2.
Aggregation into heavier fractions was also determined by the XRD of asphaltenes and coke. Two important peaks appeared at approximately 20° and 25° in the XRD patterns. It is known that the γ-band at approximately 20° includes the information of the aliphatic parts, and the (002)-band with a peak at approximately 25° indicates the stacking of aromatic sheets [6, 23, 24, 26]. The aggregation tendency of asphaltenes during the non-catalytic hydrocracking of C5-DAO is shown in Fig. 3, which indicates the XRD patterns and deconvoluted peaks of the asphaltenes obtained from the reaction for 1–8 h. It was observed that the intensity of the (002)-band increased as the reaction time increased. This implies that the aggregation of asphaltenes occurred during the non-catalytic hydrocracking reactions, as we already examined using the asphaltene feeds in our previous study [6]. The aggregation of asphaltenes was caused by the reduced steric hindrance of asphaltene molecules through the cracking of alkyl side chains [6, 5, 33–35]. The crystalline parameters of asphaltenes and coke obtained from the non-catalytic hydrocracking of C5-DAO are also summarized in Table 3. The aromaticity of asphaltenes (fa-XRD) increased significantly as the reaction time increased because of the dealkylation of side chains. The number of aromatic sheets in a stacked cluster of asphaltenes (M) seemed to remain constant because the asphaltene clusters with a high M value could be converted into coke. The aggregation tendency of coke was much higher than that of the asphaltenes obtained under the same reaction conditions. The aggregation tendency of coke also increased as the reaction time increased. These results support attributing the formation of coke during the non-catalytic hydrocracking of C5-DAO to the transformation of resins into asphaltenes followed by the formation of asphaltenes into coke through the aggregation of polyaromatic rings, even though the C5-DAO feedstock had no asphaltenes.
Roles of dispersed catalyst in catalytic hydrocracking of C5-DAO. Catalytic hydrocracking of C5-DAO was performed in the presence of 1000 ppm Mo to elucidate the effect of the catalyst on the hydrocracking of asphaltene-free feed. It has been reported that a Mo-based slurry-phase catalyst could improve the hydrogenation ability during the VR hydrocracking process and that coke formation was prevented by inhibiting the polymerization of heavy molecules into coke [37]. Coke formation was also inhibited by the dispersed catalyst when hydrocracking high-asphaltene feeds, as shown in our previous study [6]. In a similar observation, when the catalyst was applied, the asphaltene yield was less than 1 wt %, and no coke was formed even under high conversion conditions (Fig. 2). This indicates that the catalyst inhibited aggregation into heavier molecules, resulting in a significantly greater increase in the coke induction period than that in the non-catalytic hydrocracking reaction. This result is consistent with the tendency of residue conversion obtained from non-catalytic and catalytic hydrocracking summarized in Table 2. In fact, catalytic hydrocracking showed a lower residue conversion than non-catalytic hydrocracking at the initial reaction time (<4 h) because the transformation of residue into coke by polymerization occurred easily at the beginning of the reaction when the catalyst was absent. This could also be related to the rapid increase in asphaltene yield in the early part of the non-catalytic hydrocracking reaction. The gas formation in the non-catalytic hydrocracking of C5-DAO was higher than that in the catalytic hydrocracking reaction (Fig. 2). Asphaltenes were the main contributors to the formation of gases during the hydrocracking of Maya crude, followed by resins > vacuum gas oil [38]. It has been reported that the catalyst inhibited the contributions of resins and vacuum gas oil to the gas production [38]. This had good consistency with our results. It was observed that catalytic hydrocracking showed higher liquid product yield and H2 consumption than the non-catalytic hydrocracking reaction (Table 2). The H2 consumption increased significantly from 0.85 to 6.48 wt % as the reaction time increased. H2 was consumed for the termination of free radicals, hydrogenation of unsaturated bonds, and ring opening of the polyaromatic rings in catalytic hydrocracking [29, 37]. Therefore, a lower amount of resins but a higher amount of saturates were obtained in the DAO product from catalytic hydrocracking compared to those from non-catalytic hydrocracking reactions (Table 2). The SIMDIS fraction of liquid products obtained from the non-catalytic and catalytic hydrocracking of C5-DAO is also summarized in Table 2. It was observed that catalytic hydrocracking showed higher yields for middle distillate and vacuum gas oil fractions than non-catalytic hydrocracking because the free radicals driving the thermolysis could be stabilized by hydrogen. This stabilization of free radicals was facilitated by the dispersed catalyst. It was also observed that residue and DAO conversion increased at the beginning of the reaction and became almost constant. It has been reported that the conversion rate decreases as the reaction time increases in the heavy oil hydrocracking process because the rate of polyaromatic ring opening is lower than that of the dissociation or dealkylation reactions [38]. This indicates that the results corresponded well with previous reports.
The active phase of the dispersed catalyst was investigated with coke using various methods such as XRD [29, 39], XPS [7, 29], high-resolution transmission electron microscopy (HR-TEM) [7, 39–41], and extended X-ray absorption fine structure spectroscopy [41]. In this study, because almost no coke was formed during the catalytic hydrocracking reactions, activated carbon was used as an adsorbent for the Mo-based dispersed catalyst in the liquid products, as mentioned in the experimental section. Figure 4 shows the HR-TEM image and XPS Mo 3d spectra of the dispersed catalyst in activated carbon. The HR-TEM image shows crystalline layered MoS2 slabs on activated carbon with an average size of 5.4 nm (Fig. 4a). XPS Mo 3d spectroscopy of the catalyst adsorbed on the activated carbon was conducted in order to detect the main active species (Fig. 4b). The presence of three oxidation states of Mo species (Mo4+, Mo5+, and Mo6+) was observed, and Mo4+ corresponding to MoS2 was found to be the main Mo species (85.2 wt % in total Mo). These results imply that the catalyst precursor (Mo octoate) was mostly converted to MoS2, and it acted as a catalyst in the catalytic hydrocracking of C5-DAO. It has been reported that unsupported MoS2 could be formed from Mo-base precursors and S sources [42]. Since C5-DAO feedstock also had a high S content (4.3 wt %) it functioned as a S source through which to generate MoS2 during the reaction.
Product quality comparison between the non-catalytic and catalytic hydrocracking of C5-DAO provided evidence for the roles of the catalyst. Figure 5 shows the H/C ratio and MCR content of the DAO product obtained from the non-catalytic and catalytic hydrocracking of C5-DAO. The H/C ratio decreased continuously in the non-catalytic hydrocracking, indicating that more aromatic compounds remained in the DAO product as the reaction time increased. This suggests that the non-catalytic hydrocracking facilitated the dealkylation of aliphatic chains rather than the ring opening of polyaromatic rings. The polyaromatic ring compounds remaining in the DAO product seemed to cause the aggregation, which led to asphaltene and coke formation during the reaction, as shown in Fig. 2. In contrast, the H/C ratio of the DAO product obtained from the catalytic hydrocracking decreased slightly, then remained constant during the reaction. This result implies that the catalyst played a key role in enabling the saturation and ring opening of polyaromatic rings during the reaction, resulting in the formation of more aromatics and saturates (Table 2). It was observed that the MCR content of the DAO product obtained from the catalytic hydrocracking decreased significantly during the reaction. MCR is known to be a crucial factor related to coke formation [23]. This implies that the catalyst increased the conversion of heavier fractions in C5-DAO into lighter compounds, which reduced the coke precursor in the DAO product. As a result, no coke and a very small amount of asphaltenes were formed only during the catalytic hydrocracking reaction. In the absence of catalyst, the MCR content of the DAO product increased slightly and was then maintained during the non-catalytic hydrocracking reaction because the heavier asphaltenes and the coke with high MCR content were already separated from the DAO product. These results indicate that the coke formation was well controlled by the slurry-phase dispersed catalyst even in feeds with no asphaltene.
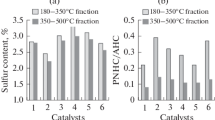
The N content of the DAO product was 0.4 wt % after catalytic hydrocracking. The N content of 0.3 wt % was in the C5-DAO feed. This indicates that the slurry-phase catalyst does not affect the removal of N. This can be explained by the fact that N is usually located inside the rings of hetero-organic compounds and was mostly not eliminated even after the hydrocracking reaction [43]. It has been reported that more hydrogen consumption is required to remove N species [44]. Unlike N species, S is located in both the aliphatic chains and rings, and the decomposition of heavy oil through the removal of S is one of the main reactions during the hydrocracking process because the S–C bond is weaker than the C–C bond [45]. It has also been reported that the removal of S (HDS) in DAO is easier than that of N (HDN) [22]. Figure 6 shows the S content change in the DAO product obtained from the non-catalytic and catalytic hydrocracking of C5-DAO. The S content in the DAO product decreased from 4.6 before to 3.4 wt % after non-catalytic hydrocracking for 8 h, whereas the S content in the DAO product obtained from the catalytic hydrocracking decreased significantly to 1.6 wt % for 8 h. These results support the consideration that the slurry-phase catalyst facilitated the removal of S from the DAO product as a result of HDS reactions.
The limitations of the abilities of the catalyst were also investigated through product quality results. The yields of resin and aromatic compounds in the DAO product were mainly constant after a long time (8–12 h). Moreover, the H/C ratio and MCR and S contents of DAO product also remained constant after long reaction times. Figure 7 shows the 13C NMR spectra of DAO product obtained from catalytic hydrocracking. The difference in aromatic carbons in the chemical shift range of 100– 150 ppm was observed. It has been reported that the chemical shift range of 118–128.5 ppm can be attributed to the presence of aromatic carbons bearing hydrogen in the triple bridge position (Car, b3), while aromatic carbons attached to alkyl side chains without CH3 (Car, alk) caused a chemical shift of 128–150 ppm [25, 46]. It is clear that the aromatic carbons were hydrocracked resulting in the removal of these peaks as the reaction time increased. The aromatic carbon peaks decreased in the initial 4 h of the reaction, then seemed to be unchanged between 8 and 12 h. This observation is consistent with the other results, as shown earlier. This could be attributed to the fact that some heavy fractions in C5-DAO were converted but still remained as a polyaromatic compound even after the catalytic hydrocracking reaction, and it took a long time to convert these heavy fractions into lighter products without coke formation.
CONCLUSIONS
The hydrocracking activities of C5-DAO were investigated under N2 and H2 pressures in the absence and presence of a Mo-octoate precursor. Thermal cracking showed a higher yield for coke than non-catalytic hydrocracking. Although the presence of H2 led to the suppression of asphaltene formation and decreased the coke yield, coke was still formed in the absence of the catalyst. The XRD patterns showed that the aggregation of heavy fractions in C5-DAO caused the formation of asphaltenes and coke during the non-catalytic hydrocracking reactions. When the dispersed catalyst was applied, the asphaltene yield was less than 1 wt % and no coke was formed even under high conversion conditions. The catalyst facilitated hydrogenation reactions and stabilized free radicals, leading to an increase in liquid production by inhibiting coke formation. The product quality was also enhanced by catalytic hydrocracking in terms of H/C ratio and MCR and S contents. Long-term testing for catalytic hydrocracking revealed the limitations on catalytic hydrogenation of polyaromatic fractions.
AUTHOR INFORMATION
Ngoc Thuy Nguyen, ORCID: https://orcid.org/0000-0001-6741-7933
Ki Hyuk Kang, ORCID: https://orcid.org/0000-0003-2638-4658
Pill Won Seo, ORCID: https://orcid.org/0000-0003-0688-9907
Narae Kang, ORCID: https://orcid.org/0000-0003-0021-4538
Duy Van Pham, ORCID: https://orcid.org/0000-0001-9526-8285
Gyoo Tae Kim, ORCID: https://orcid.org/0000-0003-0462-5000
Sunyoung Park, ORCID: https://orcid.org/0000-0003-2698-328X
REFERENCES
Castaneda, L.C., Munoz, J.A.D., and Ancheyta, J., Catal. Today, 2014, vols. 220–222, p. 248. https://doi.org/10.1016/j.cattod.2013.05.016
Zhang, S., Liu, D., Deng, W., and Que, G., Energy Fuels, 2007, vol. 21, p. 3057. https://doi.org/10.1021/ef700253f
Nguyen, M.T., Nguyen, N.T., Cho, J., Park, C.S., Jung, J., and Lee, C.W., J. Ind. Eng. Chem., 2016, vol. 43, p. 1. https://doi.org/10.1016/j.jiec.2016.07.057
Bellussi, G., Rispoli, G., Landoni, A., Millini, R., Molinari, D., Mentanari, E., Moscotti, D., and Pollesel, P., J. Catal., 2013, vol. 308, p. 189. https://doi.org/10.1016/j.jcat.2013.07.002
Calderón, C.J. and Ancheyta, J., Energy Fuels, 2016, vol. 30, p. 2525. https://doi.org/10.1021/acs.energyfuels.5b02807
Nguyen, N.T., Kang, K.H., Lee, C.W., Kim, G.T., Park, S., and Park, Y.-K., Fuel, 2019, vol. 235, p. 677. https://doi.org/10.1016/j.fuel.2018.08.035
Nguyen, N.T., Park, S., Jung, J., Cho, J., Lee, C.W., and Park, Y.-K., J. Ind. Eng. Chem., 2018, vol. 61, p. 32. https://doi.org/10.1016/j.jiec.2017.11.044
Ancheyta, J., Centeno, G., Trejo, F., and Marroquin, G., Energy Fuels, 2003, vol. 17, p. 1233. https://doi.org/10.1021/ef030023+
Trejo, F. and Ancheyta, J., Catal. Today, 2005, vol. 109, p. 99. https://doi.org/10.1016/j.cattod.2005.08.005
Trejo, F., Ancheyta, J., Centeno, G., and Marroquin, G., Catal. Today, 2005, vol. 109, p. 178. https://doi.org/10.1016/j.cattod.2005.08.013
Ancheyta, J., Centeno, G., Trejo, F., and Speight, J.G., Catal. Today, 2005, vol. 109, p. 162. https://doi.org/10.1016/j.cattod.2005.08.004
Goncalves, M.L.A., Ribeiro, D.A., Teixeira, A.M.R.F., and Teixeira, M.A.G., Fuel, 2007, vol. 86, p. 619. https://doi.org/10.1016/j.fuel.2006.08.022
Wang, J. and Anthony, E.J., Chem. Eng. Sci., 2003, vol. 58, p. 157. https://doi.org/10.1016/S0009-2509(02)00430-X
Rahmani, S. and Gray, M.R., Pet. Sci. Technol., 2007, vol. 25, p. 141. https://doi.org/10.1080/10916460601054297
Gawel, I., Bociarska, D., and Biskupski, P., Appl. Catal. A: Gen., 2005, vol. 295, p. 89. https://doi.org/10.1016/j.apcata.2005.08.001
Sámano, V., Guerrero, F., Ancheyta, J., Trejo, F., and Díaz, J.A.I., Catal. Today, 2010, vol. 150, p. 264. https://doi.org/10.1016/j.cattod.2009.09.004
Akmaz, S., Gurkaynak, M.A., and Yasar, M., J. Anal. Appl. Pyrolysis, 2012, vol. 96, p. 139.
Zhao, Y., Wei, F., and Yu, Y., J. Petrol. Sci. Eng., 2010, vol. 74, p. 20. https://doi.org/10.1016/j.petrol.2010.08.002
Douda, J., Alvarez, R., and Bolanos, J.N., Energy Fuels, 2008, vol. 22, p. 2619. https://doi.org/10.1021/ef800024p
Zhang, C., Lee, C.W., Keogh, R.A., Demirel, B., and Davis, B.H., Fuel, 2001, vol. 80, p. 1131. https://doi.org/10.1016/S0016-2361(00)00178-2
Yasar, M., Trauth, D.M., and Klein, M.Y., Energy Fuels, 2001, vol. 15, p. 504. https://doi.org/10.1021/ef0000577
Ahmed, H.S. and El-Kady, F.Y., Energy Sources, A, 2008, vol. 30, p. 247.
Prajapati, R., Kohli, K., Maity, S.K., and Garg, M.O., Fuel, 2017, vol. 203, p. 514. https://doi.org/10.1016/j.fuel.2017.04.126
Pang, W., Lee, J.-K., Yoon, S.-H., Mochida, I., Ida, T., and Ushio, M., Fuel. Process. Technol., 2010, vol. 91, p. 1517. https://doi.org/10.1016/j.fuproc.2010.05.031
Dickinson, E.M., Fuel, 1980, vol. 59, p. 290. https://doi.org/10.1016/0016-2361(80)90211-2
Yen, T.F., Erdman, J.G., and Pollack, S.S., Anal. Chem., 1961, vol. 33, p. 1587. https://doi.org/10.1021/ac60179a039
Alhumaidan, F.S., Hauser, A., Rana, M.S., Lababidi, H.M.S., and Behbehani, M., Fuel, 2015, vol. 150, p. 558. https://doi.org/10.1016/j.fuel.2015.02.076
Tanaka, R., Sato, E., Hunt, J.E., Winans, R.E., Sato, S.S., and Takanohashi, T., Energy Fuels, 2004, vol. 18, p. 1118. https://doi.org/10.1021/ef034082z
Du, H., Li, M., Liu, D., Ren, Y., and Duan, Y., Appl. Petrochem. Res., 2015, vol. 5, p. 89.
Kawai, H. and Kumata, F., Catal. Today, 1998, vol. 43, p. 281. https://doi.org/10.1016/S0920-5861(98)00157-6
Breysse, M., Furimsky, E., Kasztelan, S., Lacroix, M., and Perot, G., Catal. Rev. – Sci. Eng., 2002, vol. 44, p. 651. https://doi.org/10.1081/CR-120015483
Sun, M., Adjaye, J., and Nelson, A.E., Appl. Catal. A: Gen., 2004, vol. 263, p. 131. https://doi.org/10.1016/j.apcata.2003.12.011
Spiecker, P.M., Gawrys, K.L., Trail, C.B., and Kilpatrick, P.K., Colloids Surf. A, 2003, vol. 220, p. 9. https://doi.org/10.1016/S0927-7757(03)00079-7
Bartholdy, J. and Andersen, S.I., Energy Fuels, 2000, vol. 14, p. 52. https://doi.org/10.1021/ef990121o
Bartholdy, J., Lauridsen, R., Mejlholm, M., and Andersen, S.I., Energy Fuels, 2001, vol. 15, p. 1059. https://doi.org/10.1021/ef0100808
Ancheyta, J., Trejo, F., and Rana, M.S., Asphaltenes Chemical Transformation During Hydroprocessing of Heavy Oils, New York: CRC Press – Taylor & Francis Group, 2009.
Rezaei, H., Ardakani, S.J., and Smith, K.J., Energy Fuels, 2012, vol. 26, p. 2768. https://doi.org/10.1021/ef300034s
Ortiz-Moreno, H., Ramírez, J., Sanchez-Minero, F., Cuevas, R., and Ancheyta, J., Fuel, 2014, vol. 130, p. 263. https://doi.org/10.1016/j.fuel.2014.03.050
Rezaei, H., Liu, X., Ardakani, S.J., Smith, K.J., and Bricker, M., Catal. Today, 2010, vol. 150, p. 244. https://doi.org/10.1016/j.cattod.2009.10.005
Bellussi, G., Rispoli, G., Molinari, D., Landoni, A., Pollesel, P., Panariti, N., Millini, R., and Montanari, E., Catal. Sci. Technol., 2013, vol. 3, p. 176. https://doi.org/10.1039/C2CY20448G
Kim, S.-H., Kim, K.-D., and Lee, Y.-K., J. Catal., 2017, vol. 347, p. 127. https://doi.org/10.1016/j.jcat.2016.11.015
Sánchez, J., Moreno, A., Mondragon, F., and Smith, K.J., Energy Fuels, 2018, vol. 32, p. 7066. https://doi.org/10.1021/acs.energyfuels.8b01081
Mitra-Kirtley, S., Mullins, O.C., Elp, J.V., George, S.J., Chen, J., and Cramer, S.P., J. Am. Chem. Soc., 1993, vol. 115, p. 252. https://doi.org/10.1021/ja00054a036
Moses, P.G., J. Catal., 2015, vol. 328, p. 19. https://doi.org/10.1016/j.jcat.2014.12.019
Yen, T.F. and Chilingarian, G.V., Asphaltenes and Asphalts: Vol. 2, Amsterdam: Elsevier, 2000.
Alhumaidan, F.S., Hauser, A., Rana, M.S., and Labadili, H.M.S., Energy Fuels, 2017, vol. 31, p. 3812. https://doi.org/10.1021/acs.energyfuels.6b03433
Funding
This work was supported by the National Research Council of Science and Technology (NST) grant by the Korea government (MSIT) (no. CRC-14-1-KRICT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors declare that they have no conflict of interest.
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nguyen, N.T., Kang, K.H., Seo, P.W. et al. Hydrocracking of C5-Deasphalted Oil: Effects of H2 and Dispersed Catalysts. Pet. Chem. 61, 172–182 (2021). https://doi.org/10.1134/S0965544121020171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544121020171