Abstract
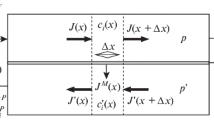
The process of unsteady-state membrane gas separation (fast-permeant impurity removal) with a pulsed retentate flow operation was considered. A semiempirical mathematical algorithm was developed to describe this process taking into account its kinetic characteristics (total cycle time, stripping time and withdrawal time, withdrawal velocity) using the MathCad® software package. Based on the developed algorithm, the basic operational parameters that affect the separation efficiency of the unsteady-state process were analyzed. It was shown that the optimal ratio of the stripping time and the withdrawal one determined by the maximum efficiency criterion more corresponds to the minimum retentate concentration than to the maximum productivity. However, the developed algorithm allows to set the productivity minimum limit by introducing additional initial data into the calculation procedure. The mathematical modeling results correlate well with the experimental data.






Similar content being viewed by others
REFERENCES
B. van der Bruggen, Ind. Eng. Chem. Res. 52, 10335 (2013).
B. Belaissaoui, Y. L. Moullec, D. Willson, and E. Favre, J. Memb. Sci. 415–416, 424 (2012).
V. M. Vorotyntsev, V. M. Malyshev, I. V. Vorotyntsev, and S. V. Battalov, Theor. Found. Chem. Eng. 50, 459 (2016).
J. Pohlmann, M. Bram, K. Wilkner, and T. Brinkmann, Int. J. Greenh. Gas Control. 53, 56 (2016).
Q. Kang, B. van der Bruggen, R. Dewil, et al., Sep. Purif. Technol. 149, 322 (2015).
P. Luis, A. Amelioa, S. Vreysen, et al., Appl. Energy. 113, 565 (2014).
C. Makhloufi, E. Lasseuguette, J. C. Remigy, et al., J. Memb. Sci. 455, 236 (2014).
C. Servel, D. Roizard, E. Favre, and D. Horbez, Ind. Eng. Chem. Res. 53, 7768 (2014).
T. S. Anokhina, A. A. Yushkin, P. M. Budd, and A. V. Volkov, Sep. Purif. Technol. 156, 683 (2015).
A. Trusov, S. Legkov, L. J. P. van den Broeke, et al., J. Memb. Sci. 383, 241 (2011).
R. W. Baker, Ind. Eng. Chem. Res. 41, 1393 (2002).
W. J. Coros, J. Memb. Sci. 83, 1 (1993).
R. Pathare and R. Agrawal, J. Memb. Sci. 364, 263 (2010).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Pet. Chem. 54, 491 (2014).
I. V. Vorotyntsev, A. A. Atlaskin, M. M. Trubyanov, et al., Desalin. Water Treat. 75, 305 (2016).
I. V. Vorotyntsev, D. N. Shablykin, P. N. Drozdov, et al., Pet. Chem. 57 (2), 172 (2017).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, and S. V. Battalov, Pet. Chem. 54, 698 (2014).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, and D. E. Tsygorov, Theor. Found. Chem. Eng. 43, 404 (2009).
D. R. Paul, Ind. Eng. Chem. Process Des. Dev. 10, 375 (1971).
L. Wang, J.-P. Corriou, C. Castel, and E. Favre, J. Memb. Sci. 383, 170 (2011).
A. Higuchi and T. Nakagawa, J. Appl. Polym. Sci. 37, 2181 (1989).
J.-P. Corriou, C. Fonteix, and E. Favre, AIChE J. 54, 1224 (2008).
X. Feng, C. Y. Pan, and J. Ivory, AIChE J. 46, 724 (2000).
Y. Chen, D. Lawless, and X. Feng, Sep. Purif. Technol. 125, 301 (2014).
D. D. Nikolić and E. S. Kikkinides, Adsorption 21, 283 (2015).
A. Shishov, A. Penkova, A. Zabrodin, et al., Talanta 148, 666 (2016).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Pet. Chem. 51, 595–600 (2011).
I. N. Beckman, A. B. Shelekhin, and V. V. Teplyakov, J. Memb. Sci. 55, 283 (1991).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Pet. Chem. 51, 492 (2011).
G. M. Howard, AIChE J. 16, 1030 (1970).
V. M. Vorotyntsev, G. M. Mochalov, M. A. Kolotilova, et al., Theor. Found. Chem. Eng. 42, 197 (2008).
V. M. Vorotyntsev, G. M. Mochalov, M. M. Trubyanov, and D. N. Shablykin, Theor. Found. Chem. Eng. 48, 55 (2014).
M. M. Trubyanov, G. M. Mochalov, V. M. Vorotyntsev, and S. S. Suvorov, Russ. J. Appl. Chem. 86, 1854 (2013).
M. M. Trubyanov, G. M. Mochalov, V. M. Vorotyntsev, and S. S. Suvorov, Sep. Purif. Technol. 135, 117 (2014).
P. N. Drozdov and I. V. Vorotyntsev, J. Theor. Found. Chem. Eng. 37, 491 (2003).
M. M. Trubyanov, P. N. Drozdov, A. A. Atlaskin, et al., J. Memb. Sci. 530, 53 (2017).
P. N. Drozdov, Y. P. Kirillov, E. Y. Kolotilov, and I. V. Vorotyntsev, Desalination 146, 249 (2002).
V. M. Vorotyntsev, P. N. Drozdov, I. V. Vorotyntsev, et al., Desalination 200, 232 (2006).
ACKNOWLEDGMENTS
The work was supported by the Russian Science Foundation, project no. 17-79-10464.
Author information
Authors and Affiliations
Corresponding author
Additional information
1The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Battalov, S.V., Sazanova, T.S., Trubyanov, M.M. et al. Modeling of Fast-Permeant Component Removal from Gas Mixture in a Membrane Module with Pulsed Retentate. Pet. Chem. 58, 806–814 (2018). https://doi.org/10.1134/S0965544118090049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544118090049