Abstract
A new assemblage of Early Vendian (Middle Ediacaran) microfossils, including acanthomorphic acritarchs and various filamentous remains, as well as sphaeromorphic striated vesicles of Valeria, which are not characteristic of deposits of this age, is described from the Ura Formation of the Dal’nyaya Taiga Group of the Patom Basin. A new species of acanthomorphs Hocosphaeridium crispum sp. nov. is recognised. The Ediacaran eukaryotic phytoplankton taphocoenoses are shown to be selectively restricted to the open-sea proximal facies of the inner ramp.
Similar content being viewed by others
INTRODUCTION
The thick (more than 2 km) sequence of postglacial deposits of the Dal’nyaya Taiga Group of the Ura Uplift contains rich fossil biotas, which mark an important stage in the diversification of eukaryotes preceding the appearance of Ediacaran organisms (Chumakov et al., 2013). The main fossils of these biotas are various acanthomorphic acritarchs, representing the phytoplankton population of the open sea basins of that time. The associations of these microfossils play a decisive role in the construction of the global Early Ediacaran biostratigraphic scale and, at the same time, provide important material for paleobiological and paleoecological reconstructions (Sergeev et al., 2011; Moczydłowska and Nagovitsin, 2012; Vorob’eva and Sergeev, 2018; Liu and Moczydłowska, 2019 ). Until recently, organic-walled microfossils in the thick section of the Dal’nyaya Taiga Group were known only from a 20-m member of greenish-gray mudstones in the uppermost part of the Ura Formation. In recent years, the paleontological record of the Dal’nyaya Taiga deposits has been noticeably expanded by finds of organic-walled microbiota in the underlying Barakun Formation (Vorob’eva and Petrov, 2020), as well as finds of macroscopic algae remains in the Ura Formation (Petrov and Vorob’eva, 2022). In this study, we present a new, unique organ-walled microbiota from the middle part of the Ura Formation, interpret the paleobiology of the fossils and the patterns of their facies-ecological distribution in the Dal’nyaya Taiga basin.
STRATIGRAPHY, AGE, AND TAPHONOMY OF THE VENDIAN FOSSIL MICROBIOTA OF THE URA UPLIFT
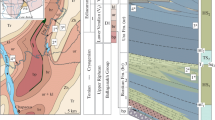
The Vendian deposits of the Ura Uplift, more than 5 km thick, accumulated in the inner (pericratonic) part of the Patom Basin, which flanked the southern margin of the Siberian Platform. The Ura Formation, 800 to 1000 m thick, is confined to the middle part of the section of postglacial deposits of the Lower Vendian Dal’nyaya Taiga Group (Fig. 1) (Chumakov et al., 2013). The formation conformably overlies the limestones of the Barakun Formation and is overlain by limestones of the Kalancha Formation with a gradual transition at the top (Petrov, 2018). Rich associations of acanthomorphic acritarchs known from the uppermost part of the section of the Ura Formation (Sergeev et al., 2011; Moczydłowska and Nagovitsin, 2012), as well as from the underlying Barakun Formation (Vorob’eva and Petrov, 2020), make it possible to correlate all these deposits with the upper part Lower Ediacaran in the interval 580–565 Ma (Liu and Moczydłowska, 2019). The top of the Dal’nyaya Taiga Group is very close in age to the beginning of the largest global δ13C Shuram anomaly in the history of the Earth (Pokrovsky and Buyakaite, 2015; Pokrovsky et al., 2021). The beginning of this event is dated at 574 ± 4.7 Ma (Rooney et al., 2020). Thus, according to modern data, the age of the Ura Formation can be estimated at 580–575 Ma. The recently obtained Pb‒Pb isochron dating of limestones of the Dal’nyaya Taiga Group, equal to 581 ± 16 and 575 ± 20 Ma (Rud’ko et al., 2021), despite a large error, generally confirms the indicated time interval. At the same time, the maximum possible age of the Barakun biota is 597 Ma.
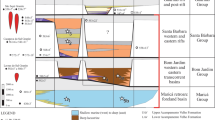
Stratigraphy, facies zones, and levels of fossil microbiota in the section of the Lower Vendian postglacial deposits of the Dal’nyaya Taiga Group of the Ura Uplift. Facies-stratigraphic levels of microbiota: (1) Barakun, after (Vorob’eva and Petrov, 2020); (2) Middle Ura, this study; (2a) level with single finds of microfossils in the most distal part of the ramp, after (Vorob’eva and Petrov, 2020); (3) Upper Ura, after (Sergeev et al., 2011; Moczydłowska and Nagovitsin, 2012), with modifications and additions.
Substantially argillaceous sediments of the Ura Formation accumulated within a deep-water (below the storm wave base) gently sloping homoclinal ramp (Petrov, 2018). In such settings, taphocenoses with the best preservation of organic-walled microfossils, as well as phytoleims of macroscopic algae, were confined to the proximal zone of the ramp, namely, to the frontal zones of progradation of carbonate platforms (Vorob’eva and Petrov, 2020; Petrov and Vorob’eva, 2022). At the foot of such platforms, the total rate of suspension and landslide sedimentation was maximum, which was the decisive factor that determined the degree of preservation of these fossil micro- and macrobiotas. The Middle Ura taphocenosis considered in this paper is confined to the distal zone of the inner ramp (Fig. 1). Among the sedimentary textures of this facies zone, there are no signs of either large-scale slope landslide or gravity flow deposits. However, the increased amount of fine-grained siliciclastics in comparison with the underlying and overlying mudstones indicates a short-term and, probably, local increase in the rates of suspension sedimentation, which provided a reasonable state of preservation of the Middle Ura fossil microfossils.
MATERIAL AND METHODS
The studied material comes from a small outcrop on the right bank of the Ura River, 8.5 km upstream of its mouth (GPS: 60.280333° N, 117.1344° E). The section with an apparent thickness of 26 m is represented by an alternation of gray and greenish-gray mudstones and silty mudstones with occasional beds of black siltstones and fine-grained sandstones. Some beds contain a small amount of carbonate. These deposits occur in the middle part of the Ura Formation, 360 m above its base and about 500 m below the top of the formation within the western limb of the Ura Anticline. A total of 26 samples were taken, 17 samples contained identifiable fossils. Laboratory sample preparation and maceration of organic-walled microfossils from silty mudstones, as well as the preparation of permanent preparations, were carried out according to the standard method described in our previous publication (Vorob’eva and Petrov, 2020). The macerate of all studied samples containing microfossils contained a large amount of highly decomposed organic matter as finely dispersed masses and structureless sapropel-like films up to 2 mm across. In general, the preservation of the studied microfossils can be assessed as satisfactory. Most of the identified forms have signs of intense corrosion, obviously associated with postmortal syn- and early diagenetic bacterial destruction. In this case, the vesicles of acanthomorphic microfossils are largely destroyed, but processes are well preserved.
The microfossils were photographed in transmitted light using a Zeiss Axio Imager A1 microscope equipped with an Axio-CamMRc-5 digital camera and an RME-5 microscope equipped with a Canon EOS 300D digital camera. All material is housed in the Geological Institute of the Russian Academy of Sciences (Moscow), paleontological collection no. 14715.
MIDDLE URA MICROBIOTA
In the studied association of morphologically diverse microfossils (Pls. I–VI), spheromorphic acritarchs are the most numerous group of microfossils. They were found in all samples containing any identifiable microfossils at most levels of the section where samples were taken. Many spheromorphites represent the species Leiosphaeridia crassa (Naumova) emend. Jankauskas, L. jacutica (Timofeev) emend. Mikhailova et Jankauskas, L. minutissima (Naumova) emend. Jankauskas, and L. tenuissima Eisenack (40% of the total sample of microfossils). These forms include vesicles 150–250 µm in diameter, with characteristic annular and sickle-shaped creases (Pl. III, figs. 9, 11). These vesicles can formally be attributed to the taxon L. jacutica, however, the regular concentric arrangement of the folds brings these forms closer to Simia nerjenica A. Weiss. At the same time, the absence of a discoid internal body does not allow us to attribute these microfossils to this taxon. Another remarkable and relatively numerous (about 3% of all microfossils) group of spheromorphic acritarchs is Valeria aff. lophostriata (Jankauskas) (Pl. II). The genus Valeria Jankauskas emend. Nagovitsin has a very wide stratigraphic and geographic distribution. To date, the oldest finds of V. lophostriata are known from the Ruyang group with an age of 1.75–1.4 Ga, China (Pang et al., 2015), and in the Mallapunyah Formation, 1.65 Ga, Australia (Javaux et al., 2004), while the youngest are from the Chuar Group, 0.78–0.74 Ga, Grand Canyon, Arizona (Riedman and Porter, 2016). In the deposits of the Vanavara Formation, which belongs to the lower Upper Vendian (or to the middle part of the Ediacaran), only one find of a small (70 µm) striated vesicle cf. Valeria lophostriata (Nagovitsin and Kochnev, 2015, p. 754, text-fig. 4.20). However, the quality of the presented material does not allow us to confidently attribute this form to this taxon. Thus, the Ura striated vesicles represent the first and most numerous finds of such acritarchs in Lower Vendian deposits. The significantly increased stratigraphic interval of their distribution confirms the suggestion of X. Hofmann (Hofmann, 1999) that Valeria may represent a whole class of organisms, and not just one biological species. Various filamentous microfossils make up the most significant (more than 45%) part of all microfossils found. Filament bundles of Siphonophycus robustum (Schopf) emend. Knoll et Golubic, comb. Knoll, Swett et Mark (Pl. IV, fig. 3) and S. typicum (Hermann) comb. Butterfield are common. These forms usually form colonies of randomly intertwined filaments over 1 mm long. Other species are also abundant in the association: S. solidum (Golub) comb. Butterfield, S. punctatum Maithy, and also Oscillatoriopsis obtusa Schopf emend. Butterfield (Pl. IV, figs. 4a, 4b). Polytrichoides lineatus Hermann (pl. I, fig. 14) and single specimens of Glomovertella rotundata (Kolosov) comb. Vorob’eva et Petrov (Pl. IV, figs. 1, 2) are less common. A large number of microfossils are large structureless films, as well as tubular and ribbon-like thalli, which can be straight, sometimes curved, or twisted into a long flat spiral (Pl. IV, figs. 9–13). In the association, ribbon-like thalli of Tyrasotaenia podolica (Gnilovskaya) (Pl. IV, figs. 15, 17) and Vanavarataenia insolita Pjatiletov (Pl. IV, figs. 5–8) are relatively rare. Together with them, there are single presumed fungal forms; Caudosphaera expansa Hermann et Timofeev, as well as an unidentified two-layered thallus with swellings, inside which are putative sporangium-like structures (Pl. IV, fig. 16). The association contains single taxonomically indeterminate remains of possible multicellular organisms (Pl. III, figs. 7, 10). These fragments are preserved in the form of films with cells significantly larger than in previously described similar forms. Similar remains found in cherts of the Doushantuo Formation in China have been interpreted as possible florideophytes (Ouyang et al., 2021; Shang and Liu, 2022). Acanthomorphic acritarchs make up about 10% of the entire sample of microfossils. The association is dominated by Knollisphaeridium coniformum Liu et Moczydłowska (7.5%) (Pl. III, figs. 1–5) and Hocosphaeridium crispum sp. nov. (2.8%) (Pl. I). The latter form, judging by the published data (Grazhdankin et al., 2020), is almost identical to the specimen diagnosed as Mengeosphaera sp. from the post-Ediacaran deposits of the Nemakit-Daldynian Stage (Kessyusa Group) of the north of the Siberian Platform. The Middle Ura microfossil association also contains a few Appendisphaera tenuis Moczydłowska, Vidal et Rudavskaya, emend. Moczydłowska, Eotylotopalla strobilata (Faizullin) and Tanarium digitiformum (Nagovitsin et M. Faizullin) Sergeev, Knoll et Vorob’eva.
In general, the studied Middle Ura microfossil association shows a relatively impoverished set of acanthomorphic acritarchs compared to both the Upper Ura (Sergeev et al., 2011; Moczydłowska and Nagovitsin, 2012) and the underlying Barakun Formation (Vorob’eva and Petrov, 2020) associations. At the same time, the association of acanthomorphites of this association is dominated by the species Hocosphaeridium crispum sp. nov. On the other hand, a characteristic feature of the Middle Ura association is the predominance of various filamentous forms, as well as spheromorphic acritarchs, among which numerous striatal sheaths of Valeria stand out, which are not known in other associations of the Dal’nyaya Taiga Group.
FACIES-STRATIGRAPHIC DISTRIBUTION OF MICROFOSSILS IN THE DAL’NYAYA TAIGA PALEOBASIN
A comparative analysis of the taxonomic composition of various associations of microfossils of the Barakun and Ura formations (Fig. 1) suggests interpretation of the paleoecological structure of the communities of the Dal’nyaya Taiga paleobasin. Generally, the differences in the taxonomic composition between different-aged and heterofacies associations of fossil organisms are determined by three parameters: the rate of evolutionary radiation of the groups of organisms under consideration, the facies area of their habitat and the area of their dispersion, all other taphonomic conditions being equal. Here we consider three groups of such organisms. The first group consists of the Early Ediacaran eukaryotic phytoplankton, represented by various acanthomorphic Doushantuo-Pertatataka acritarchs. Facies analysis allows distinguishing of taxa of narrow and relatively wide distribution. Widely zoned representatives include only three species—Appendisphaera tenuis, Knollisphaeridium maximum (Yin), emend. Willman et Moczydłowska and K. coniformum, while all the rest have a very narrow range of distribution, limited by the proximal zone of the inner ramp. The only exception is Hocosphaeridium crispum sp. nov., confined to the distal facies of the Middle Ura association (Fig. 1). The second group is represented by cyanobacterial communities, most of which have the widest range of distribution, and only two species, Rugosoopsis tenuis Timofeev et Hermann, emend. Butterfield, Knoll et Swett and Segmentothallus asperus Hermann are restricted to proximal facies (Fig. 1). The third group includes forms of micro- and macroalgal, as well as supposed fungal nature. In this group, the remains of previously described macrophytes Liulingjitaenia Chen et Xiao and Jiuqunaoella Chen emend. Xiao, Yuan, Steiner et Knoll (Petrov and Vorob’eva, 2022) are confined to the proximal zone, while the fungiform taxa Vanavarataenia insolita and the algal thalli Tyrasotaenia podolica are present only in the distal facies of the Middle Ura association (Fig. 1). All these associations of acanthomorphs of the Dal’nyaya Taiga Group reflect a single Middle Ediacaran stage in the evolution of eukaryotic phytoplankton. Most of these microfossils, as well as macrophyte remains, are confined to the facies of the proximal part of the inner ramp against the background of a wide distribution of planktonic and benthic cyanobacterial communities. Obviously, all these organisms constituted a specific paleoecological system of the open sea basins of that time. Mass appearance in the Middle Ura interval of atypical representatives of Vanavarataenia, Tyrasotaenia, and striated vesicles of Valeria, which are more characteristic of shallow-water facies (Hofmann, 1999), could be related to the transfer of these remains from the coastal zone of the basin. Probably atypical acanthomorphic vesicles of Hocosphaeridium crispum sp. nov. have the same origin. Such interzonal transport was quite possible in the absence of extensive carbonate platforms that had not yet formed in Middle Ura time. On the whole, the facies trend of distribution of microfossil associations of the Dal’nyaya Taiga basin is part of the general trend from shallow water basins of the interior regions of the Siberian Platform to deep water pericratonic basins of the Patom Zone (Nagovitsin and Kochnev, 2015).
SYSTEMATIC PALEONTOLOGY
INCERTAE SEDIS
GROUP MICROFOSSILS
SUBGROUP ACRITARCHA EVITT, 1963
Genus Hocosphaeridium Zang in Zang et Walter, 1992 emend. Xiao, Zhou, Liu, Wang et Yuan, 2014
Hocosphaeridium crispum Vorob’eva et Petrov, sp. nov.
Plate I, figs. 1–9
Mengeosphaera sp.: Grazhdankin et al., 2020, Fig. 4E.
Etymology. From the Latin crispus (curly).
Holotype. GIN RAS, specimen no. 14715-885, preparation 21040-6, site 4, Baikal-Patom Highlands, Ura River, 8.5 km upstream of its mouth, right bank (GPS: 60.280333° N, 117.1344° E), Ura Formation, Dal’nyaya Taiga Group, Vendian.

Plate I . Hocosphaeridium crispum sp. nov. (1, 1a) specimen GIN, no. 14715-871, preparation 21040-4, site 15, (1a) magnified fragment; (2, 2a) holotype, specimen GIN, no. 14715-885, preparation 21040-6, site 4, (2a) magnified fragment; (3) specimen GIN, no. 14715-908, preparation 21040-8, site 10; (4, 4a, 4b) specimen GIN, no. 14715-952, preparation 21040-15, site 6, (4a, 4b) magnified fragments; (5, 5a) specimen GIN, no. 14715-876, preparation 21040-5, site 5, (5a) magnified fragment; (6, 6a) specimen GIN, no. 14715-839, preparation 21040-1, site 6, (6a) magnified fragment; (7, 7a) specimen GIN, no. 14715-706, preparation 21038-11, site 18, (7a) magnified fragment; (8, 8a) specimen GIN, no. 14715-841, preparation 21040-1, (8, 8a) magnified fragment; (9) specimen GIN, no. 14715-372, preparation 21037-3, site 6. The sample numbers correspond to the first five digits in the preparation numbers.
Diagnosis. Large vesicle with numerous closely spaced large homomorphic processes with hooked terminations. Processes hollow, communicating with the vesicle cavity and basally separated from each other. Expanded base of the processes passing into a wide tube, which gradually tapers to a pointed, hook-shaped termination. Processes bent basally by 90°–270°.
Description. The vesicles are oval and spherical, densely covered with large homomorphic processes. The size of vesicles varies from 240 × 325 to 325 × 485 µm (average 291 × 396 µm; holotype 220 × 430 µm). Length of processes from 50 to 90 µm (average 71 µm, 19% of shell diameter; holotype from 75 to 82 µm, 23%). Base of processes are 11–22 µm wide (16 µm on average, holotype 15–17 µm) passes into a straight tube 9–13 µm wide (10 µm on average, holotype 10–12 µm). The end of the tube narrows to 2–4 µm and bends basally, sometimes almost forming a ring. The number of processes along the circumference of the vesicle varies from 60 to 150 (the holotype is more than 120).
Comparison. H. crispum is distinguished from all other species by larger vesicle sizes and a greater number of processes. It differs from H. anozos (Willman) Xiao, Zhou, Liu, Wang et Yuan in larger processes, and from H. dilatatum Liu, Xiao, Yin, Chen, Zhou et Li in wider processes.
Remarks. The shape of the processes base may vary slightly in different specimens, sometimes within the same form. In some processes, the bases can be only slightly widened and can gradually taper towards the main process tube. Some processes have a highly expanded conical base with a flexure that becomes a tube.
Occurrence. Ura Formation, Baikal-Patom Highlands, Vendian; Oppokun Formation, Nemakit-Daldynian Stage, Lena-Anabar Depression, Lower Cambrian.
Material. 13 complete specimens of satisfactory preservation and 18 incomplete fragments of poor preservation.
Genus Knollisphaeridium Willman et Moczydłowska, 2008, emended Liu et Moczydłowska, 2019
Knollisphaeridium coniformum Liu et Moczydłowska, 2019
Plate III, figs. 1–5
Knollisphaeridium maximum (Yin, 1987) Willman et Moczydłowska, 2008: Xiao et al., 2014, p. 30, text-figs. 19.1–19.7; Sergeev et al., 2011, p. 995, text-fig. 7.5.
Knollisphaeridium coniformum Liu et Moczydłowska, 2019: Liu and Moczydłowska, 2019, pp. 115–118, text-figs. 62A–62H; Ouyang et al., 2021, p. 20, text-figs. 15A–15F, 15H–15G; Ye et al., 2022, p. 39, text-fig. 27.
Holotype. Specimen IGCAGS-NP1-197, Yangtze Gorge Region, South China, NP6-4-14, J37/2; cherty concretions of the Doushantuo Formation, Member II of the Chenjiayuanzi section.
Description. Large spherical vesicles 170–475 µm in diameter, bearing numerous, uniformly and closely spaced processes of the same length. The processes are hollow, communicating with the internal cavity of the vesicles. The processes have an expanded base, with a slight bend turning into a thin termination. The bases of the processes are close, but not connected. Length of processes is 4–10 µm, width of bases 3–5 µm, distance between the bases 1–5 µm, number of processes 10–20 per 50 µm along the contour of the vesicles
Remarks. Almost all processes in all specimens of K. coniformum have terminal extensions less than 1 µm in diameter, often funnel-shaped, which are not typical for the diagnosis of this genus (Pl. III, figs. 3c, 5b). Only a few processes do not have such extensions. Extensions could be both a morphological feature of the organism and the result of taphonomy. The single pointed ends of the processes are more likely the result of the loss of the extended part during burial rather than the acquisition of such extensions during fossilization. The submicron size of the objects does not allow us to confirm this.
Comparison. It differs from K. maximum in more numerous and densely spaced processes, which gradually, without inflection, narrow from base to apex.
Occurrence. Doushantuo Formation, Hubei Province, South China, Ediacaran; Ura Formation, Baikal-Patom Highlands, Russia, Vendian.
Material. 82 specimens of satisfactory preservation.
Genus Valeria Jankauskas, 1982, emend. Nagovitsin, 2009
Valeria aff. lophostriata (Jankauskas, 1979) Jakauskas, 1982
Plate II, figs. 1–8
Valeria aff. lophostriata (Jankauskas). (1, 1a) specimen GIN, no. 14715-355, preparation 21037-2, site 1, (1a) magnified fragment; 2, (2a) specimen GIN, no. 14715-357, preparation 21037-2, site 3, (2a) magnified fragment; (3, 3a) specimen GIN, no. 14715-384, preparation 21037-5, site 2, (3a) magnified fragment; (4, 4a, 4b) specimen GIN, no. 14715-809, preparation 21038-21, site 6, (4a, 4b) magnified fragments; (5, 5a) specimen GIN, no. 14715-540, preparation 21037-17, site 7, (5a) magnified fragment; (6, 6a) specimen GIN, no. 14715-971, preparation 21040-18, site 7, (6a) magnified fragment; (7, 7a) specimen GIN, no. 14715-365, preparation 21037-2, site 11, (7a) magnified fragment; (8, 8a, 8b) specimen GIN, no. 14715-446, preparation 21037-9, site 2, (8a, 8b) magnified fragments. The sample numbers correspond to the first five digits in the preparation numbers.
Microfossils from the Ura Formation. (1–5) Knollisphaeridium coniformum Liu, Moczydłowska: (1, 1a) specimen GIN, no. 14715-188, preparation 20006-10, site 1, (1a) magnified fragment; (2, 2a) specimen GIN, no. 14715-168, preparation 20006-8, site 1, (2a) magnified fragment; (3, 3a–3c) specimen GIN, no. 14715-243, preparation 20006-20, site 3, (3a–3c) magnified fragments; (4, 4a) specimen GIN, no. 14715-912, preparation 21040-9, site 4, (4a) magnified fragment; (5, 5a, 5b) specimen GIN, no. 14715-854, preparation 21040-3, site 6a, (5a, 5b) magnified fragments; (6) Tanarium digitiformum (Nagovitsin et M. Faizullin) Sergeev, Knoll et Vorob’eva: specimen GIN, no. 14715-299, preparation 20008-1, site 1; (7, 7a, 10) Gen. et sp. indet. 1: (7, 7a) specimen GIN, no. 14715-367, preparation 21037-3, site 1, (7a) magnified fragment; (10) specimen GIN, no. 14715-237, preparation 20006-19, site 6; (8) Eotylotopalla strobilata (Faizullin), specimen GIN, no. 14715-140, preparation 20006-3, site 2; (9, 11) L. jacutica (Timofeev) emend. Mikhailova et Jankauskas: (9) specimen GIN, no. 14715-665, preparation 21038-8, site 5; (11) specimen GIN, no. 1471520, preparation 17131-3, site 2. The sample numbers correspond to the first five digits in the preparation numbers.
Kildinella lophostriata Jankauskas, 1979: Jankauskas, 1979, p. 192, text-figs. 1.13–1.15; Volkova, 1981, p. 71, pl. 8a, 8b.
Agidelia reta Pjatiletov et Karlova, 1980: Pjatiletov and Karlova, 1980, pp. 63, 65, 126, pl. 2, fig. 11.
Agidelia lophostriata Pjatiletov, 1980: Pjatiletov, 1980, p. 74, pl. 5, figs. 1, 2.
Kildinosphaera lophostriata (Jankauskas) Vidal, 1983: Vidal and Siedlecka, 1983, p. 59, text-figs. 6A–6G; Vidal and Knoll, 1983, text-figs. 1D, 1E; Vidal and Ford, 1985, p. 361, text-figs. 4C, 4E–4F.
Valeria lophostriata (Jankauskas, 1979) Jankauskas, 1982: Jankauskas, 1982, p. 109, pl. 39, fig. 2; Jankauskas et al., 1989, p. 86, pl. 16, figs. 2–5; Butterfield and Chandler, 1992, pp. 948–949, text-figs. 5A, 5B; Hofmann and Jackson, 1994, p. 24, pl. 17, figs. 14–15; pl. 19, fig. 4; Xiao et al., 1997, p. 201, text-fig. 3e; Veiss et al., 1998, p. 25, pl. 1, figs. 18, 19; Samuelsson, 1997, p. 180, text-figs. 10B, 10C; Samuelsson et al., 1999, p. 15, text-fig. 8E; Javaux et al., 2001, p. 67, text-fig. 1D; Javaux et al., 2003, p. 125, text-figs. 1.1–2; Knoll et al., 2006, p. 1026, text-figs. 2d, 2e; Lamb et al., 2009, p. 97, Fig. 4a; Nagy et al., 2009, p. 2, text-figs. 1A, 1B; Nagovitsin, 2009, p. 144, Fig. 4E; Stanevich et al., 2009, p. 9, pl. 2, figs. 5, 11, 12; Pang et al., 2015, p. 255, text-fig. 3; Tang et al., 2015, p. 308, text-fig. 11; Wellman and Strother, 2015, p. 16, text-fig. 3I; Riedman and Porter, 2016, p. 10, text-fig. 4.1; Porter and Riedman, 2016, p. 842, text-figs. 19.1–19.3; Baludikay et al., 2016, p. 174, text-fig. 7H; Javaux and Knoll, 2017, p. 219, text-figs. 7.1–7.4; Agić et al., 2017, p. 119, text-fig. 12-I; Adam et al., 2017, p. 388, text-figs. 3D–3E; Beghin et al., 2017, p. 72, pls. 4j–4k; Javaux and Lepot, 2018, p. 72, text-figs. 2a, 2b; Loron et al., 2018, p. 3, text-figs. 1C–1D; Miao et al., 2019, p. 194, text-figs. 11a–11f; Strother and Wellman, 2021, p. 6, text-figs. 3a, 3b; Sharma et al., 2021, text-fig. 7C; Loron et al., 2021, p. 6, text-fig. 4.1; Shuvalova et al., 2021, p. 44, text-figs. 3g, 3h;
Valeria aff. lophostriata Jankauskas: Gladkochub et al., 2009, text-fig. 3, figs. 10, 11, 13.
Holotype. LitNIGRI, no. 16-62-4762/16, copyspecimen 1; Bashkir Urals, Kabakovo-62 Borehole, interval 4762–4765 m, Upper Riphean.
Description. Large ellipsoidal vesicles, from 180 × 120 to 450 × 325 µm in diameter, with surface ornamentation of evenly and closely spaced concentric ridges. The width of the ridges is about 1 µm, the height of the ridges is less than 1 µm, the distance between the ridges is from 0.8 to 1.3 µm. The walls of the vesicles are thin, translucent, with numerous crumpling folds. There is no striation at the poles. Some vesicles show a medial cleavage oriented along the ridges.
Comparison. The distinctly pronounced striations of the vesicles makes it possible to identify them as representatives of the genus Valeria. However, the vesicles found by us differ from the characteristic representatives of V. lophostriata in the absence of pronounced striations at the poles, which can be both a biological characters and a consequence of the poor preservation of the material.
Material. 13 intact specimens and 18 poorly preserved fragments.
Occurrence. V. lophostriata has a wide stratigraphic occurrence, from Late Paleoproterozoic (Javaux et al., 2004; Agić et al., 2017; Miao et al., 2019) to Cryogenian (Nagy et al., 2009). V. lophostriata was also found in the presumably Lower Cambrian Gouhou Formation of North China (Tang et al., 2015; He et al., 2017). We found Valeria aff. lophostriata in the Ura Formation, the Ediacaran age of which is now confirmed.
FAMILY OSCILLATORIACEAE (S.F. GRAY) KIRCHNER, 1900
Genus Glomovertella Reitlinger, 1948
Glomovertella rotundata (Kolosov, 1984) comb. Vorob’eva et Petrov, 2022
Plate IV, figs. 1, 2
Microfossils from the Ura Formation. (1, 1a, 2) Glomovertella rotundata (Kolosov) comb. Vorob’eva et Petrov: (1) specimen GIN, no. 14715-790, preparation 21038-20, site 1, (1a) magnified fragment; (2) specimen GIN, no. 14715-266, preparation 20006-22, site 12; (3) Siphonophycus robustum (Schopf) emend. Knoll et Golubic, comb. Knoll, Swett et Mark, specimen GIN, no. 14715-375, preparation 21037-3, site 9; (4) Oscillatoriopsis obtusa Schopf, emend. Butterfield, specimen GIN, no. 14715-949, preparation 21040-15, site 3, (4a) magnified fragment; (5–8) Vanavarataenia insolita Pjatiletov: (5) specimen GIN, no. 14715-191, preparation 20006-10, site 4; (6) specimen GIN, no. 14715-813, preparation 21038-21, site 11; (7) specimen GIN, no. 14715-464, preparation 21037-11, site 1; (8) specimen GIN, no. 14715-153, preparation 20006-5, site 3; (9–13) structureless tubular and ribbon-like thalli: (9) specimen GIN, no. 14715-166, preparation 20006-7, site 4; (10) specimen GIN, no. 14715-825, preparation 21039-1, site 8; (11) specimen GIN, no. 14715-301, preparation 20008-2, site 2; (12) specimen GIN, no. 14715-66, preparation 17138-6, site 4; (13) specimen GIN, no. 14715-744, preparation 21038-16, site 1; (14) Polytrichoides lineatus Hermann, specimen GIN, no. 14715-490, preparation 21037-13, site 4b; (15, 17) Tyrasotaenia podolica (Gnilovskaya): (15) specimen GIN, no. 14715-633, preparation 21038-2, site 11; (17) specimen GIN, no. 14715-1039, preparation 21042-2, site 10; (16) Gen. et sp. indet. 2, specimen GIN, no. 14715-67, preparation 17138-6, site 5. The sample numbers correspond to the first five digits in the preparation numbers.
Volyniella rotundata Kolosov, 1984: Kolosov, 1984, pl. XIV, figs. 3a, 3b, pl. XV, fig. 1.
Volyniella torta Kolosov, 1984: Kolosov, 1984, pl. XIV, fig. 2.
Glomovertella rotundata (Kolosov, 1984) comb. Kolosov, 1989: Jankauskas et al., 1989, pl. XLV, fig. 1.
Circumiella torta (Kolosov, 1984) comb. Kolosov, 1989: Jankauskas et al., 1989, pl. XLV, fig. 2.
Glomovertella rotundata (Kolosov, 1984) comb. Vorob’eva et Petrov, 2022: Vorob’eva and Petrov, 2023, pl. I, figs. 1, 2, 4, 6.
Holotype. YaF GS SB RAS, no. 87-101, preparation 565-80/1, c.s. 39.8:119.5; Eastern Siberia, Western Yakutia, Srednebotuobinsk Field, Borehole 23, depth 1911.5–1915.2 m; Vendian, Kursov Formation; Kolosov, 1984, pl. XV, fig. 1.
Description. Tubular filaments with a width of 15 to 42 microns, coiled into 2–3 planispiral whorls. The size of the spiral is from 230 to 400 µm. Some of the filaments have segmentation, the length of the segments is 1–4 µm.
Comparison. This species differs from G. ampla Yakschin in the thinner filaments. It is distinguished from G. eniseica (Hermann), G. glomerata (Jankauskas), and G. miroedikhia Hermann in the larger size.
Occurrence. Kursov, Byuk, Zherba formations, Eastern Siberia, Upper Vendian. Ura Formation, Baikal–Patom Highlands, Vendian.
Material. Nine poorly preserved specimens.
FAMILY VENDOTAENIACEAE GNILOVSKAYA, 1986
Genus Tyrasotaenia Gnilovskaya, 1971
Tyrasotaenia podolica (Gnilovskaya, 1971)
Plate IV, figs. 15, 17
Tyrasotaenia podolica Gnilovskaya, 1971: Gnilovskaya, 1971, p. 106, pl. XI, figs. 1–5; Gnilovskaya, 1976, pp. 11–12; Gnilovskaya, 1979, p. 41, pl. XLIV, figs. 4, 6; Gnilovskaya, 1985, p. 120, pl. 34, figs. 1, 3, 4; Gnilovskaya et al., 1988, pl. IX, fig. 4, pl. XIV, figs. 1–4; Zaine, 1991, pl. 10; Leonov et al., 2009, p. 92, text-fig. E-7; Cohen et al., 2009, p. 120, text-fig. 9.3; Ragozina et al., 2016, p. 32, pl. 1.1.
Holotype. IGGD RAS, no. 6931/1; Podolsk Transnistria, Kanilov Group, Studenitskaya Formation, Vendian.
Description. Isolated long ribbon-like films, thin, translucent, with longitudinal folds, curved and twisted. Film length ranges from 0.45 to 1.4 mm, width from 0.08 to 0.12 mm.
Occurrence. Studenitskaya Formation, Podolsk Transnistria, Ukraine, Vendian. Moldova: Ferapontiev Formation, Upper Vendian; Tigech Formation, Lower Cambrian. Dabis Formation, Nama Group, Namibia, late Ediacaran. Tamengo Formation, Corumba Group, Brazil, Ediacaran. Tsaganolom Formation, Western Mongolia, Upper Vendian. Ura Formation, Baikal–Patom Highlands, Russia, Vendian.
Material. Nine specimens in a satisfactory state of preservation.
Genus Vanavarataenia Pjatiletov 1985
Vanavarataenia insolita Pjatiletov 1985
Plate IV, figs. 5–8
Vanavarataenia insolita Pjatiletov, 1985: Pjatiletov, 1985, p. 935, figs. 1a–1e; Jankauskas et al., 1989, p. 134, pl. 46, figs. 7, 8; Marusin et al., 2011, p. 662, text-fig. 3d; Nagovitsin and Kochnev, 2015, p. 754, text-fig. 4, figs. 33, 34, 37; Golubkova et al., 2020, p. 104, pl. 9, fig. 7.
Holotype. IGiG SB RAS. Siberian Platform, upper reaches of the Podkamennaya Tunguska River, Borehole Sobinskaya no. 10, Vanavara Formation, Vendian.
Description. Fragments of unbranched thalli, 30–70 µm wide, straight or curved. The width of the thalli is variable, may vary throughout or decrease towards the ends. The length of the fragments reaches 750 µm. The thalli bear lateral and terminal sporangium-like structures, spherical vesicles ranging in size from 40 × 25 to 140 × 100 µm. Lateral vesicles are solitary, sessile, closely adjacent to the thallus. One thallus may contain one terminal and several lateral membranes of different sizes, which may correspond to the stages of growth of sporangium-like structures.
Comparison. It has been suggested that the similarity of the main morphological elements of the structure and size in the genera Vanavarataenia Pjatiletov and Vendomyces Burzin may indicate that they may belong to the same genus (Vorob’eva and Petrov, 2014) or to be identical (Nagovitsin and Kochnev, 2015). An analysis of the material published and available to us shows an obvious difference between these taxa. Compared to Vendomyces, in Vanavarataenia the “sporangia” are always solitary, they do not form clusters, they have neither a neck nor opening structures, and the thalli themselves never branch.
Occurrence. Upper subformation of the Vasileostrovskaya Formation, East European Platform, Upper Vendian. Vanavara Formation, Siberian Platform, Vendian. Starorechenskaya Formation, Anabar Uplift of Siberia, Upper Vendian. Ura Formation, Baikal–Patom Highlands, Vendian.
Material. Twenty-four species in a satisfactory state of preservation.
Gen. et sp. indet. 1
Plate III, figs. 7, 10
Unnamed multicellular form with relatively large cells: Ouyang et al., 2021, p. 11, text-fig. 9I.
Unnamed species B: Shang, Liu, 2022, p. 16, text-fig. 13.
Description. Fragments of single-layer films, consisting of cells tightly adjacent to each other. The shape of the cells is trapezoidal, five- and hexagonal. Cells tend to be densely packed. The cell walls are thin and transparent. The cell size is from 45 × 40 to 80 × 40 µm. The film is up to 300 µm across.
Comparison. This species differs from other described multicellular and colonial forms by a larger cell size.
Occurrence. Doushantuo Formation, Hubei Province, South China, Ediacaran. Ura Formation, Baikal–Patom Highlands, Russia, Vendian
Material. Two specimens.
Gen. et sp. indet. 2
Plate IV, fig. 16
Description. Tubular thallus of irregular width, has local swellings and constrictions, smooth, without septa. The specimen is 360 µm long, 10 µm wide at the constrictions, widened up to 65 µm in places of swelling. The thallus is two-layered; the top layer is a thin film that tightly envelops the internal contents. Inside the swelling there is a spherical body (sporangium?) with a diameter of 50 µm, which does not completely occupy the internal space.
Occurrence. Ura Formation, Baikal–Patom Highlands, Vendian.
Material. One specimen.
CONCLUSIONS
In the middle part of the section of the Ura Formation, a new representative association of Early Vendian (Middle Ediacaran) microfossils was found, among which there are forms unknown in the previously studied fossil microbiota of the Dal’nyaya Taiga basin. Large (up to 450 µm) striatal spheromorphic vesicles of Valeria and densely ornamented acanthomorphic vesicles of Hocosphaeridium crispum sp. nov. up to 330 µm. The fungiform Vanavarataenia insolita and thalli of Tyrasotaenia podolica are also atypical representatives of the Middle Ura association. Comparative analysis of the taxonomic composition of associations of acanthomorphic acritarchs at different stratigraphic levels of the Dal’nyaya Taiga Group indicates the absence of any pronounced evolutionary trend. All these associations reflect a single Middle Ediacaran stage in the evolution of eukaryotic phytoplankton and can be considered within a single complex biozone. Most representatives of Ediacaran eukaryotes in the studied taphocenoses gravitated towards relatively narrow facies zones of the inner ramp in comparison with the cyanobacterial communities widespread in the basin. Both of them made up the paleoecological system of the open sea basins of that time. The appearance of atypical representatives in the Middle Ura microbiota could be associated with their transfer from the shallow water zones of the basin, in particular, from the inner basins of the Siberian craton.
REFERENCES
Adam, Z.R., Skidmore, M.L., Mogk, D.W., and Butterfield, N.J., A Laurentian record of the earliest fossil eukaryotes, Geology, 2017, vol. 45, pp. 387–390. https://doi.org/10.1130/G38749.1
Agić, H., Moczydłowska, M., and Yin, L., Diversity of organic-walled microfossils from the early Mesoproterozoic Ruyang Group, North China Craton—A window into the early eukaryote evolution, Precambrian. Res., 2017, vol. 297, pp. 101–130. https://doi.org/10.1016/j.precamres.2017.04.042
Baludikay, B.K., Storme, J.Y., Francois, C., Baudet, D., and Javaux, E.J., A diverse and exquisitely preserved organic-walled microfossil assemblage from the Meso-Neoproterozoic Mbuji-Mayi Supergroup (Democratic Republic of Congo) and implications for Proterozoic biostratigraphy, Precambrian. Res., 2016, vol. 281, pp. 166–184. https://doi.org/10.1016/j.precamres.2016.05.017
Beghin, J., Storme, J.Y., Blanpied, C., Gueneli, N., Brocks, J.J., Poulton, S.W., and Javaux, E.J., Microfossils from the late Mesoproterozoic–early Neoproterozoic Atar/El Mreïti Group, Taoudeni Basin, Mauritania, northwestern Africa, Precambrian. Res., 2017, vol. 291, pp. 63–82. https://doi.org/10.1016/j.precamres.2017.01.009
Butterfield, N.J. and Chandler, F.W., Palaeoenvironmental distribution of Proterozoic microfossils, with an example from the Agu Bay Formation, Baffin Island, Palaeontology, 1992, vol. 35, pp. 943–957.
Chumakov, N.M., Semikhatov, M.A., and Sergeev, V.N., Vendian reference section of southern Middle Siberia, Stratigr. Geol. Correl., 2013, vol. 21, no. 4, pp. 359–382. https://doi.org/10.7868/S0869592X13040029
Cohen, P.A., Bradley, A., Knoll, A.H., Grotzinger, J.P., Jensen, S., Abelson, J., Hand, K., Love, G., Metz, J., McLoughlin, N., Meister, P., Shepard, R., Tice, M., and Wilson, J.P., Tubular compression fossils from the Ediacaran Nama Group, Namibia, J. Palaeont., 2009, vol. 83(1), pp. 110–122. https://doi.org/10.1666/09-040R.1
Gladkochub, D.P., Stanevich, A.M., Travin, A.V., Mazukabzov, A.M., Konstantinov, K.M., Yudin, D.S., and Kornilova, T.A., The Mesoproterozoic Udzha paleorift (Northern Siberian Craton): New data on age of basites, straigraphy, and microphytology, Dokl. Earth Sci., 2009, vol. 425, pp. 371–377.
Gnilovskaya, M.B., The most ancient water plants of the Vendian of the Russian platform (Late Precambrian), Paleont. Zh., 1971, no. 3, pp. 101–107.
Gnilovskaya, M.B., Ancient Metaphyta, in Mezhd. geol. kongr. XXV sess. Dokl. sov. geologov. Paleontologiya. Morskaya Geologiya (Proc. XXV Sess. Int. Geol. Congr. Reports of Soviet Geologists. Paleontology, Marine Geology), Moscow, 1976, pp. 10–14.
Gnilovskaya, M.B., Vendotenides, in Paleontologiya verkhnedokembriiskikh i kembriiskikh otlozhenii Vostochno-Evropeiskoi platformy (Paleontology of the Upper Precambrian and Cambrian Sediments of the East European Platform), Moscow: Nauka, 1979, pp. 39–48.
Gnilovskaya, M.B., Vendotenides—Vendian Metaphyta, in Vendskaya sistema. T. 1 (Vendian System. Vol. 1), Moscow: Nauka, 1985, pp. 117–125.
Gnilovskaya, M.B., Ishchenko, A.A., Kolesnikov, Ch.M., Korenchuk, L.V., and Udal’tsov, A.P., Vendotenidy Vostochno-Evropeiskoi platformy (Vendotenides of the East European Platform), Leningrad: Nauka, 1988 [in Russian].
Golubkova, E.Yu., Kushim, E.A., and Tarasenko, A.B., Fossil organisms of the Kotlin Regional Stage of the Upper Vendian of the Northwestern Russian Platform (Leningrad Region), Paleontol. J., 2020, vol. 54, pp. 420–428. https://doi.org/10.31857/S0031031X20040066
Grazhdankin, D., Nagovitsin, K., Golubkova, E., Karlova, G., Kochnev, B., Rogov, V., and Marusin, V., Doushantuo-Pertatataka-type acanthomorphs and Ediacaran ecosystem stability, Geology, 2020, vol. 48. https://doi.org/10.1130/G47467.1
He, T., Zhou, Y., Vermeesch, P., Rittner, M., Miao, L., Zhu, M., Carter, A., Pogge von Strandmann, P.A.E., and Shields, G.A., Measuring the ‘Great Unconformity’ on the North China Craton using new detrital zircon age data, Spec. Publ.—Geol. Soc. London, 2017, vol. 448, pp. 145–159. https://doi.org/10.1144/SP448.14
Hofmann, Y.J., Global distribution of the Proterozoic sphaeromorph acritarch Valeria lophostriata (Jankauskas), Acta Micropalaeontol. Sinica, 1999, vol. 16, no. 3, pp. 215—224 (in Chinese).
Hofmann, H.J. and Jackson, C.D., Shelf-facies microfossils from the Proterozoic Bylot Supergroup, Baffin Island, Canada, Paleontol. Soc. Mem., 1994, vol. 37, no. 3, pp. 361–382. https://doi.org/10.1017/S0022336000030353
Javaux, E.J. and Knoll, A.H., Micropaleontology of the lower Mesoproterozoic Roper Group, Australia, and implications for early eukaryotic evolution, J. Palaeontol., 2017, vol. 91, pp. 199–229. https://doi.org/10.1017/jpa.2016.124
Javaux, E.J. and Lepot, K., The Paleoproterozoic fossil record: Implications for the evolution of the biosphere during Earth’s middle-age, Earth-Sci. Rev., 2018, vol. 176, pp. 68–86. https://doi.org/10.1016/j.earscirev.2017.10.001
Javaux, E.J., Knoll, A.H., and Walter, M.R., Ecological and morphological complexity in early eukaryotic ecosystems, Nature, 2001, vol. 412, pp. 66–69. https://doi.org/10.1038/35083562
Javaux, E.J., Knoll, A.H., and Walter, M.R., Recognizing and interpreting the fossils of early eukaryotes, Origins of Life and Evolution of the Biosphere, 2003, vol. 33, pp. 75–94. https://doi.org/10.1023/a:1023992712071
Javaux, E.J., Knoll, A.H., and Walter, M.R., TEM evidence for eukaryotic diversity in mid-Proterozoic oceans, Geobiology, 2004, vol. 2, pp. 121–132. https://doi.org/10.1111/j.1472-4677.2004.00027.x
Knoll, A.H., Javaux, E.J., Hewitt, D., and Cohen, P., Eukaryotic organisms in Proterozoic oceans, Phil. Trans. R. Soc. Bull., 2006, vol. 361, pp. 1023–1038. https://doi.org/10.1098/rstb.2006.1843
Kolosov, P.N., Pozdnedokembriiskie mikroorganizmy vostoka Sibirskoi platformy (Late Precambrian Microorganisms from the Eastern Siberian Platform), Yakutsk: Yakutsk. Fil. Sib. Otd. Akad. Nauk SSSR, 1984 [in Russian].
Lamb, D.M., Awramik, S.M., Chapma, D.J., and Zhu, S., Evidence for eukaryotic diversification in the ∼1800 million-year-old Changzhougou Formation, North China, World Sum. on Ancient Microscopic Fossils, 2009, vol. 173, pp. 93–104. https://doi.org/10.1016/j.precamres.2009.05.005
Leonov, M.V., Fedonkin, M.A., Vickers-Rich, P., Ivantsov, A.Yu., and Trusler, P., Discovery of the first macroscopic carbonaceous algal assemblage in the Terminal Proterozoic of Namibia, southwest Africa, Communs Geol. Surv. Namibia, 2009, vol. 14, pp. 87–93.
Liu, P. and Moczydłowska, M., Ediacaran microfossils from the Doushantuo Formation chert nodules in the Yangtze Gorges area, South China, and new biozones, Fossils and Strata, 2019, vol. 65, pp. 1–172. https://doi.org/10.1002/9781119564225.ch1
Loron, C.C., Rainbird, R.H., Turner, E.C., Greenman, J.W., and Javaux, E.J., Implications of selective predation on the macroevolution of eukaryotes: Evidence from Arctic Canada, Emerging Topics in Life Sciences, 2018, vol. 2, pp. 247–255. https://doi.org/10.1042/ETLS20170153
Loron, C.C., Halverson, G.P., Rainbird, R.H., Skulski, T., Turner, E.C., and Javaux, E.J., Shale-hosted biota from the Dismal Lakes Group in Arctic Canada supports an early Mesoproterozoic diversification of eukaryotes, J. Palaeontol., 2021, vol. 95, no. 6, pp. 1113–1137. https://doi.org/10.1017/jpa.2021.45
Marusin, V.V., Grazhdankin, D.V., and Maslov, A.V., Redkino stage in evolution of Vendian macrophytes, Dokl. Earth Sci., 2011, vol. 436, pp. 197–202. https://doi.org/10.31857/S2686739721110128
Miao, L., Moczydłowska, M., Zhu, S., and Zhu, M., New record of organic-walled, morphologically distinct microfossils from the late Paleoproterozoic Changcheng Group in the Yanshan Range, North China, Precambrian Res., 2019, vol. 321, pp. 172–198. https://doi.org/10.1016/j.precamres.2018.11.019
Moczydłowska, M. and Nagovitsin, K.E., Ediacaran radiation of organic-walled microbiota recorded in the Ura Formation, Patom Uplift, East Siberia, Precambrian Res., 2012, vol. 198-199, pp. 1–24. https://doi.org/10.1016/j.precamres.2011.12.010
Nagovitsin K.E. Tappania-bearing association of the Siberian platform: Biodiversity, stratigraphic position and geochronological constraints, Precambrian Res., 2009, vol. 173, pp. 137–145. https://doi.org/10.1016/j.precamres.2009.02.005
Nagovitsin, K.E. and Kochnev, B.B., Microfossils and biofacies of the Vendian fossil biota in the southern Siberian Platform, Russ. Geol. Geophys., 2015, vol. 56, no. 4, pp. 584–593. https://doi.org/10.15372/GiG20150409
Nagy, R.M., Porter, S.M., Dehler, C.M., and Shen, Y., Biotic turnover driven by eutrophication before the Sturtian low-latitude glaciation, Nature Geosci., 2009, vol. 2, pp. 415–418. https://doi.org/10.1038/ngeo525
Ouyang, Q., Zhou, C., Xiao, S., Guan, C., Chen, Z., Yuan, X., and Sun, Y., Distribution of Ediacaran acanthomorphic acritarchs in the lower Doushantuo Formation of the Yangtze Gorges area, South China: Evolutionary and stratigraphic implications, Precambrian Res., 2021, vol. 353. 106005. https://doi.org/10.1016/j.precamres.2020.106005
Pang, K., Tang, Q., Yuan, X.L., Wan, B., and Xiao, S., A biomechanical analysis of the early eukaryotic fossil Valeria and new occurrence of organic-walled microfossils from the Paleo-Mesoproterozoic Ruyang Group, Palaeoworld, 2015, vol. 24, pp. 251–262. https://doi.org/10.1016/j.palwor.2015.04.002
Petrov, P.Yu., Postglacial deposits of the Dal’nyaya Taiga Group: Early Vendian in the Ura Uplift, Siberia. Communication 2. Ura and Kalancha formations and history of the basin, Lithol. Miner. Resour., 2018b, vol. 53, no. 6, pp. 473–488. https://doi.org/10.1134/S0024497X18060083
Petrov, P.Yu. and Vorob’eva, N.G., Representatives of the Miaohe biota from the Ediacaran (Vendian) pre-Shuram strata of the Patom Highland, Siberia, Stratigr. Geol. Correl., 2022, vol. 30, no. 1, pp. 52–64. https://doi.org/10.31857/S0869592X22010069
Pokrovsky, B.G. and Buyakaite, M.I., Geochemistry of C, O, and Sr isotopes in the Neoproterozoic carbonates from the southwestern Patom paleobasin, southern Middle Siberia, Lithol. Miner. Resour., 2015, vol. 50, no. 2, pp. 144–169. https://doi.org/10.7868/S0024497X15010048
Pokrovsky, B.G., Buyakaite, M.I., Kolesnikova, A.A., Petrov, O.L., and Khlebnikov, M.S., C, O, and Sr isotope geochemistry of the Vendian Shuram–Wonoka anomaly and associated metasedimentary rocks in the inner part of the Patom Upland (Central Siberia), Lithol. Miner. Resour., 2021, vol. 56, no. 5, pp. 390–417. https://doi.org/10.31857/S0024497X21050049
Porter, S.M. and Riedman, L.A., Systematics of organic-walled microfossils from the ca. 780–740 Ma Chuar Group, Grand Canyon, Arizona, J. Palaeontol., 2016, vol. 90, pp. 815–853.
Pyatiletov, V.G., Microphytofossils from Late Precambrian deposits, penetrated by the Vanavara borehole (the western Siberian platform), in Novye dannye po stratigrafii pozdnego dokembriya zapada Sibirskoi platformy i ee skladchatogo obramleniya (New Data on the Late Precambrian Stratigraphy of the Western Siberian Platform and its Folded Mountain Frame), Novosibirsk: Inst. Geol. Geofiz. Sib. Otd. Akad. Nauk SSSR, 1980, pp. 71–76.
Pyatiletov, V.G., Yudomian (Vendian) algae of the western Siberian Platform, Dokl. Akad. Nauk SSSR, 1985, vol. 281, no. 4, pp. 934–936.
Pyatiletov, V.G. and Karlova, G.A., The Upper Riphean assemblage of plant micro fossils of the Yenisei Ridge, in Novye dannye po stratigrafii pozdnego dokembriya zapada Sibirskoi platformy i ee skladchatogo obramleniya (New Data on the Late Precambrian Stratigraphy of the Western Siberian Platform and its Folded Mountain Frame), Novosibirsk: Inst. Geol. Geofiz. Sib. Otd. Akad. Nauk SSSR, 1980, pp. 56–71.
Ragozina, A.L., Dorjinamjaa, D., Serezhnikova, E.A., Zaitseva, L.V., and Enkhbaatar, B., Association of macro- and microfossils in the Vendian (Ediacaran) postglacial successions in Western Mongolia, Stratigr. Geol. Correl., 2016, vol. 24, no. 3, pp. 242–251. https://doi.org/10.31857/S0031031X22040092
Riedman, L.A. and Porter, S., Organic-walled microfossils of the mid-Neoproterozoic Alinya Formation, Officer Basin, Australia, J. Palaeontol., 2016, vol. 90, pp. 854–887.
Rooney, A.D., Cantine, M.D., Bergmann, K.D., Gomez-Perez, I., Al Baloushi, B., Boag, T.H., Busch, J.F., Sperling, E.A., and Strauss, J.V., Calibrating the coevolution of Ediacaran life and environment, Proc. Natl. Acad. Sci. U.S.A., 2020, vol. 117, no. 29, pp. 16824–16830. https://doi.org/10.1073/pnas.2002918117
Rud’ko, S.V., Kuznetsov, A.V., Petrov, P.Yu., Sitkina, D.R., and Kaurova, O.K., Pb–Pb dating of the Dal’nyaya Taiga Group in the Ura uplift of southern Siberia: implication of C-isotopic and biotic events in the Ediacaran, Precambrian Res., 2021, vol. 362, 106285. https://doi.org/10.1016/j.precamres.2021.106285
Samuelsson, J., Biostratigraphy and Palaeobiology of Early Neoproterozoic Strata of the Kola Peninsula, Northwest Russia, Norsk Geol. Tidsskr., 1997, vol. 77, pp. 165–192.
Samuelsson, J., Dawes, P.R., and Vidal, G., Organic-walled microfossils from the Proterozoic Thule Supergroup, Northwest Greenland, Precambrian Res., 1999, vol. 96, pp. 1–23. https://doi.org/10.1016/S0301-9268(98)00123-5
Sergeev, V.N., Knoll, A.H., and Vorob’eva, N.G., Ediacaran microfossils from the Ura Formation, Baikal–Patom Uplift, Siberia: Taxonomy and biostratigraphic significance, J. Paleontol., 2011, vol. 85, no. 5, pp. 987–1011. https://doi.org/10.1666/11-022.1
Shang, X. and Liu, P., Diverse multicellular algae from the early Ediacaran Doushantuo chert nodules and their palaeoecological implications, Precambrian Res., 2022, vol. 368, 106508. https://doi.org/10.1016/j.precamres.2021.106508
Sharma, M., Singh, V.K., Pandey, S.K., Ansari, A.H., Shukla, Y., Ahmad, S., Kumar, Y., and Singh, D., Precambrian and early Cambrian palaeobiology of India: Quo Vadis, Proc. Indian Nat. Sci. Acad., 2021, vol. 87, pp. 199–233.
Shuvalova, Yu.V., Nagovitsin, K.E., and Parkhaev, P.Yu., Evidences of the oldest trophic interactions in the Riphean biota (Lakhanda Lagerstätte, Southeastern Siberia), Dokl. Biol. Sci., 2021, vol. 496, pp. 34–40. https://doi.org/10.31857/S2686738921010200
Stanevich, A.M., Gladkochub, D.P., Kornilova, T.A., Mazukabzov, A.M., and Karmanov, N.S., Microphytofossils of the Riphean Udzha Formation of the north of Siberian craton, Izv. Tomsk. Politekhn. Univ., 2009, vol. 315, no. 1, pp. 5–10.
Strother, P.K. and Wellman, C.H., The Nonesuch Formation Lagerstätte: A rare window into freshwater life one billion years ago, J. Geol. Soc., 2021, vol. 178. https://doi.org/10.1144/jgs2020-133
Tang, Q., Pang, K., Yuan, X., Wan, B., and Xiao, S., Organic-walled microfossils from the Tonian Gouhou Formation, Huaibei region, North China Craton, and their biostratigraphic implications, Precambrian. Res., 2015, vol. 266, pp. 296–318. https://doi.org/10.1016/j.precamres.2015.05.025
Veis, A.F., Petrov, P.Yu., and Vorob’eva, N.G., The Late Riphean Miroedikha microbiota from Siberia. Part 1: Composition and facial-ecological distribution of organic-walled microfossils, Stratigr. Geol. Korrel., 1998, vol. 6, no. 5, pp. 15–37.
Vidal, G. and Ford, T.D., Microbiotas from the late Proterozoic Chuar Group (northern Arizona) and Uinta Mountain Group (Utah) and their chronostratigraphic implications, Precambrian. Res., 1985, vol. 28, pp. 349–389. https://doi.org/10.1016/0301-9268(85)90038-5
Vidal, G. and Knoll, A.H., Proterozoic plankton, Geol. Soc. Am. Mem., 1983, vol. 161, pp. 265–277.
Vidal, G. and Siedlecka, A., Planktonic, acid-resistant microfossils from the Upper Proterozoic strata of Barents Sea region of Varanger Peninsula, East Finnmark, Northen Norway, Norg. Geol. undersøkelse Bull., 1983, vol. 382, pp. 45–79.
Volkova, H.A., Acritarchs from the Upper Precambrian of southeastern Siberia (Ust’kirba Formation), Byull. Mosk. O–va Ispyt. Prir., Otd. Geol., 1981, vol. 56, no. 4, pp. 66–75.
Vorob’eva, N.G. and Petrov, P.Yu., The genus Vendomyces Burzin and facies-ecological specificity of the Staraya Rechka microbiota of the Late Vendian of the Anabar Uplift of Siberia and its stratigraphic analogues, Paleontol. Zh., 2014, vol. 48, pp. 655–666. https://doi.org/10.7868/S0031031X14060166
Vorob’eva, N.G. and Petrov, P.Yu., Microbiota of the Barakun Formation and biostratigraphic characteristics of the Dal’nyaya Taiga Group: Early Vendian of the Ura Uplift (Eastern Siberia), Stratigr. Geol. Correl., 2020, vol. 28, no. 4, pp. 365–380. https://doi.org/10.31857/S0869592X20040109
Vorob’eva, N.G. and Petrov, P.Yu., Microfossils and Sedimentary Environments of the Zherba Basin: Upper Vendian of the Patom Highland of Siberia, Stratigr. Geol. Correl., 2023, vol. 31, no. 2, pp. 17-32.
Vorob’eva, N.G. and Sergeev, V.N., Stellarossica gen. nov. and the Infragroup Keltmiides infragroup. nov.: Extremely Large Acanthomorph Acritarchs from the Vendian of Siberia and the East European Platform, Paleontol. J., 2018, no. 5, pp. 563–573. https://doi.org/10.1134/S0031031X18040141
Wellman, C.H. and Strother, P.K., The terrestrial biota prior to the origin of land plants (embryophytes): A review of the evidence, Palaeontology, 2015, pp. 1–27. https://doi.org/10.1111/pala.12172
Willman, S. and Moczydłowska, M., Ediacaran acritarch biota from the Giles 1 drillhole, Officer Basin, Australia, and its potential for biostratigraphic correlation, Precambrian Res., 2008, vol. 162, pp. 498–530. https://doi.org/10.1016/j.precamres.2007.10.010
Xiao, S., Knoll, A.H., Kaufman, A.J., Yin, L., and Zhang, Y., Neoproterozoic fossils in Mesoproterozoic rocks? Chemostratigraphic resolution of a biostratigraphic conundrum from the North China Platform, Precambrian Res., 1997, vol. 84, pp. 197–220. https://doi.org/10.1016/S0301-9268(97)00029-6
Xiao, S., Zhou, C., Liu, P., Wang, D., and Yuan, X., Phosphatized acantomorphic acritarchs and related microfossils from the Ediacaran Doushauntuo Formation at Weng’an (South Chine) and their implications for biostratigraphic correlation, J. Paleontol., 2014, vol. 88, no. 1, pp. 1–67. https://doi.org/10.1666/12-157R
Yankauskas, T.V., Microbiota of the Southern Urals and Bashkir Cis-Urals, Dokl. Akad. Nauk SSSR, 1979, vol. 248, no. 1, pp. 190–193.
Yankauskas, T.V., Riphean microfossils of the Southern Urals, in Stratotip rifeya. Paleontologiya. Paleomagnetizm (The Stratotype of Riphean. Paleontology. Paleomagnetism), Moscow: Nauka, 1982, pp. 84–120.
Yankauskas, T.V., Mikhailova, N.S., German, T.N., Sergeev, V.N., et al., Mikrofossilii dokembriya SSSR (Precambrian Microfossils of the USSR), Leningrad: Nauka, 1989 [in Russian].
Ye, Q., Li, J., Tong, J., An, Z., Hu, J., and Xiao, S., A microfossil assemblage from the Ediacaran Doushantuo Formation in the Shennongjia area (Hubei Province, South China): Filling critical paleoenvironmental and biostratigraphic gaps, Precambrian Res., 2022, vol. 377, 106691. https://doi.org/10.1016/j.precamres.2022.106691
Zaine, M.F., Analise dos Fósseis de Parte da Faixa Paraguai (MS, MT) e seu Contexto Temporal e Paleoambiental. Tese de Dourado, São Paulo: Instituto de Geociências, 1991.
Zang, W.-L. and Walter, M.R., Late Proterozoic and Early Cambrian microfossils and biostratigraphy, Amadeus Basin, Central Australia, Mem. Assoc. Australasian Palaeontol., 1992, vol. 12.
Funding
The microphytological studies were conducted in accordance with the plans of the research work of the Geological Institute of the Russian Academy of Sciences, project no. FMMG-2023-0004 (Vorob’eva N.G.), facies studies were carried out at the expense of the Russian Science Foundation grant No. 20-77-10066 (Petrov P.Yu.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by S. Nikolaeva
>Reviewers E.A. Luzhnaya, A.L. Ragozina, and M.A. Fedonkin
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vorob’eva, N.G., Petrov, P.Y. Middle Ura Association of Organic-Walled Microfossils: the Lower Vendian of the Patom Basin, Siberia. Stratigr. Geol. Correl. 31, 410–424 (2023). https://doi.org/10.1134/S086959382305009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S086959382305009X