Abstract
Phase equilibria were studied experimentally in three rock samples of the Ary-Bulak massif at T = 800–700°C, P = 1 kbar, water content 10 wt %, and oxygen fugacity corresponding to the Ni–NiO and Mt–Hem buffers. Liquidus phases in melts obtained by melting of Ca-rich ongonites are fluorite, topaz, quartz, and plagioclase (andesine, bytownite). The liquid immiscibility of silicate and F–Ca salt melts described in the Ary-Bulak ongonites was not found in the experimental samples. The liquidus temperature of Ca-rich samples is ≥800°C, which is not typical for highly evolved differentiated granite melts. Based on the obtained factual material, it was concluded that simple experiments on melting–crystallization of rock samples of the Ary-Bulak massif do not fully reproduce its formation. It is possible that an important role in nature was played by irreversible processes that are not taken into account in this experimental series: interaction with host rocks, a sharp significant change in the fluid regime or P-T parameters, etc. The relicts of immiscible silicate and salt F–Ca melts described in natural Ca–F-rich samples also could be caused by one of these processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Ongonites are subvolcanic analogues of Li–F topaz-bearing granites, which are potentially ore-bearing rocks. The study of ongonites provides insight into magmatic stage of their formation (Kovalenko and Kovalenko, 1976; Beskin et al., 1994; Zaraisky et al., 2009). The rare-metal Li–F granites were generated from a residual acidified granitic melt during simultaneous crystallization of minerals from melt and fluid under elevated fluorine activity (Kovalenko and Kovalenko, 1976). At the postmagmatic stage, the highly evolved granites could be significantly reworked by metasomatic solutions with transfer and subsequent redeposition of components and formation of new mineral phases. The scales of this reworking have remained unclear yet. Subvolcanic bodies are less subjected to low temperature metasomatic processes and their rocks retain better primary magmatic appearance. Therefore, the determination of conditions of ongonite formation is of great importance for the complex study of the genesis of Li–F granites.
Experimental studies revealed the wide immiscibility fields in fluoride–silicate melts. Thereby, one of the melts is usually represented by fluorides and aluminofluorides of alkali or alkali earth metals. Addition of these components causes heterogeneity in silicate melts owing to the formation of sybotaxic groups enriched in fluorine and network-modifier cations (Kogarko and Krigman, 1981). In many fluoride–silicate systems, melts show supraliquidus microheterogeneity (immiscible splitting). According to Anfilogov et al. (1990, 2005), the immiscible splitting at a microscopic scale is considered as the formation of thermodynamically stable critical melt emulsions. These authors believe that the silicate–salt liquid immiscibility was caused by inconsistency between ion structure of typical salts and ion-covalent structure of SiO2. Experimental data (Gramenitskii et al., 2005, Alferyeva et al., 2020a) showed that the formation of fluoride melts at the magmatic stage could affect the distribution of ore components and, finally, serves as one of the decisive factors for ore deposition.
It was shown that some ongonites of the Ary-Bulak massif differ from the typical ongonites in the high F and Ca contents and the presence of fluorite. According to (Kovalenko and Kovalenko, 1976), the late glass from porphyritic ongonites of this massif shows a negative correlation between F and Ca content, on the one hand, and Si, Al, and sometimes alkali elements, on the other. It was assumed that the glass is crystallized into Ca- and F-rich phase (fluorite type) and Al-rich phase with low Si content. The experimental study of melting and crystallization of the granites in the presence of HF solution revealed that saturation of granitic melt in fluorine produces in addition to silicate melt, Al silicofluoride melt sharply enriched in Ca compared to silicate melt. Kovalenko and Kovalenko (1976) emphasized that such compositional heterogeneities of glass are typical of ongonites of the Ary-Bulak massif.
The study of melt and fluid inclusions, as well as structural–textural features of the rocks allowed Peretyazhko et al. (2007) to establish the existence of fluoride–calcium melt during crystallization of ongonites of the Ary-Bulak massif. This melt has coexisted with a residual aluminosilicate melt at temperature below β → α-quartz transition (595–585°С). Cross-cutting veinlets in aphyric rocks filled with “fluorite” phase bearing microinclusions of prosopite CaAl2(F,OH)8 and aqueous calcium aluminofluorides also indicates the presence of Al- and fluid-rich fluoride–calcium melts. It was proposed that the high concentrations of oxygen dissolved in the F–Ca melt prevented the crystallization of fluorite throughout the entire magmatic stage. It was concluded (Peretyazhko and Savina, 2010a, 2010b) that ongonite magma in addition to crystalline phases and silicate melts contained aqueous–salt fluids of different types, as well as diverse immiscible fluoride melts that are close in component proportions to fluorite, sellaite, cryolite, chiolite, and more complex aluminofluoride composition. The authors suggest that some residual silicate melts had an elevated alkalinity (A/CNK < 1) and very high fluorine concentrations (7–15 wt %), and at temperature below 700–720°C they were split into immiscible silicate melts (A/CNK =1) and drops of cryolite-like and other fluoride melts. Thus, ongonite magma within a temperature range of 700–600°C likely contained segregations of diverse fluoride melts rather than round fluoride crystals (cryolite, fluorite, sellaite, chiolite). It is suggested that the formation of marginal zone of F- and Ca-rich aphyric and porphyritic rocks was caused by the local decompression of magmatic chamber. The decompression led to quenching of immiscible aluminosilicate and fluoride (mainly F–Ca) melts at the crystallization front and formation of aphyric rock. The aphyric rocks experienced autometasomatic alteration under the influence of magmatic NaF-bearing fluid of P–Q type, which was exsolved during degassing of a residual ongonite melt in the central part of the massif. The possible reasons of the elevated Ca and Sr contents in the Ary-Bulak ongonite magma were discussed in (Peretyazhko et al., 2011). The authors argue against the partial assimilation of limestones of the Ust-Borzya Formation by magma based on the find of high-Sr Ca-rich ongonites, the absence of carbonate xenoliths, the relatively low Sr contents in the limestones, as well as the low CO2 contents in primary fluid inclusions. We believe that this question has not yet been solved and requires further study. According to Peretyazhko et al., fluoride melts are produced only by fluoride–silicate liquid immiscibility, while the enrichment of ongonite varieties of the Ary-Bulak massif in Ca and Sr is seeming due to their insignificant volume.
A different point of view on the origin of enriched in Ca, K, and F inner contact facies of the Ary-Bulak massif was proposed by Antipin et al. (2009), who suggested that such unusual chemical composition of ongonites, in particular, the high Ca and Sr contents, could be related to the possible assimilation of limestones, which are localized at a depth and belong to the Ust-Borzya Formation hosting the Ary-Bulak massif. The formation of most part of the Ary-Bulak rocks was determined by magmatic differentiation of crustal granite magma. The geochemical evolution of rare-metal ongonite magmatism of the Ary-Bulak massif is a sufficiently difficult process and likely involves assimilation of host limestones by a common magma at the early stage magmatic and further differentiation of the melt in a subsurface chamber.
The relicts of the F–Ca melt were found not only in ongonites (Peretyazhko et al., 2018; Peretyazhko and Savina, 2020), but also in rhyolites and granite pegmatites (Vasyukova and Williams-Jones, 2014), as well as in carbonatites (Potter et al., 2017). In particular, the fluid–silicate immiscibility has existed at the magmatic stage of the formation of rhyolites of the Nyalga Basin in Central Mongolia (Peretyazhko et al., 2018а). It is suggested that the immiscible splitting of F–Ca melt began in local domains of magmatic chamber, when F content in the rhyolite melt reached 1.5–2 wt %. The F–Ca melt has the elevated contents of oxygen, REE, Y, Sr, P, Sc, and likely, aqueous fluid. Fluorite did not crystallize in this melt. The F–Ca melt has existed in a flowing state prior to magma eruption at the surface. The influence of fluid released during degassing and crystallization of rhyolite melt on the Ca- and F-rich rocks led to the partial removal of trace elements from a quenched F–Ca phase during its transformation in fluorite. It remains unclear yet why F–Ca melt is not crystallized in fluorite in rhyolite magma within a wide temperature range up to its vitrification. This was likely caused by the high oxygen fugacity, the presence of aqueous fluid, and elevated temperatures. At the same time, Peretyazhko et al. in some cases suggest the formation of fluorite and associated ore mineralization from F–Ca melt at the fluid-magmatic stage.
The liquid immiscibility in the CaF2-granite system at atmospheric pressure was confirmed experimentally (Yang and van Hinsberg, 2019). At 1200°C, the immiscibility is encountered at F content more than 4.4 wt %, which decreases to 0.75 wt % at temperature decrease below 900°C. The presented phase diagram shows the possible coexistence of oxysilicate, fluorosilicate melts, and fluorite within temperature range from 1100–1200 to 600–700°C, below which only oxysilicate melt and fluorite exist. Rapid cooling prevents the nucleation of fluorite, while the forming metastable fluorosilicate melt has elevated concentrations of Ca and F and can be preserved up to 500°C.
A F–Ca melt was obtained experimentally within a wide P-T range (5.5–1 kbar, 1250–750°C) by melting of the fluorite-rich rhyolites of the Nyalga Basin in Central Mongolia (Peretyazhko et al., 2018b; Suk et al., 2018; Peretyazhko et al., 2020). The fluoride–silicate liquid immiscibility was observed at F > 2.5–5 wt % and CaO > 5.3 wt % in the system. As temperature and pressure decrease from 1250 to 900°C and from 5.5 to 1 kbar, the immiscibility field is widened; the compositions of coexisting phases become more contrasting, and fluorite begins to crystallize. The stability field of silicate and fluoride–calcium melts, and fluorite likely exist at T = 750°C and P = 1 kbar in the presence of 10 wt % H2O2 solution. A temperature decrease to 650°C likely leads to the formation of crystalline fluorite. At eruption of rhyolitic magma of the Nyalga Basin, the oxygen-bearing F–Ca melt likely exists in a metastable supercooled state under oxidizing conditions at oxygen fugacity above Ni–NiO buffer (Peretyazhko and Savina, 2020).
Our experimental works were aimed at studying phase relations and searching for immiscibility between silicate and F–Ca salt melts at melting of ongonites of the Ary-Bulak massif under subliquidus conditions.
GEOLOGICAL POSITION AND PETROGRAPHY OF THE ROCKS OF THE ARY-BULAK MASSIF
The Ary-Bulak massif is located in the Borzya district of Eastern Transbaikalia. It represents a zonal laccolith. Host rocks are the limestones, metavolcanic rocks, schists, and sandstones of the J3–K1 Ust-Borzya Formation. The age of the Ary-Bulak massif is 142.1 ± 0.7 Ma according to (Kostitsyn et al., 1995) and 141.6 ± 0.5 Ma with allowance for Ca- and F-rich rocks of the massif according to (Peretyazhko et al., 2011).
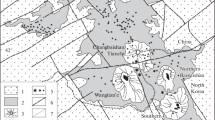
At the modern erosion level, the massif has an oval shape 900 × 500 m in size (Fig. 1). The central part of the massif is made up of white porphyritic ongonites, which according to (Kovalenko and Kovalenko, 1976; Kovalenko et al., 1999; Antipin et al., 2009) are replaced to flanks by porphyritic fluorite–topaz ongonites with elevated contents of CaО (5.4 wt %) and F (5.2 wt %). Peretyazhko and Savina (2010b) report that the massif has “a spotted structure”. The porphyritic varieties with extremely high CaO and F concentrations form bodies up to a few square meters in area within porphyritic ongonites and in appearance are almost indistinguishable from common porphyritic ongonites. It was also noted that these bodies occur near the southwestern flank of the massif and more rarely in its central part and on the northeastern flank. The inner contact zone of the southwestern part is made up of aphyric rocks, which contain up to 22 and 19 wt % calcium and fluorine, respectively (Peretyazhko and Savina, 2010b).
Geological scheme of the Ary-Bulak massif after (Antipin et al., 2009). (1) Quaternary deposits; rocks of the Ary-Bulak massif: (2) coarsely porphyritic ongonites of the central facies, (3) ongonites of the marginal facies, (4) quenched fine-grained and glassy, sometimes fluidal rocks of the inner contact facies, (5) subalkaline basalts, basaltic andesites, (6) weakly metamorphosed limestones, (7) schists, crystalline schists, (8) periodically drying out lake Ongonite. Inset shows the position of the Ary-Bulak massif in Transbaikalia.
The detailed petrographic description and chemical composition of the massif rocks are reported in (Kovalenko et al., 1971; Kovalenko and Kovalenko, 1976; Antipin et al., 2009; Peretyazhko et al., 2007; Peretyazhko and Savina, 2010a, 2010b). Therefore, we give only brief petrographic characteristics. Ongonites in the central part of the massif have a porphyritic texture. The porphyric phenocrysts compose 45–50% of the rock and include quartz (10%), albite (15%), sanidine (15%), topaz (5%), and zinnwaldite mica (1%). The micrograined groundmass is made up of the same minerals. In the fluorite-bearing porphyritic ongonites, the content of porphyry phenocrysts significantly decreases (20–30%). The groundmass contains submicron intergrowths of “fluorite” and “K-feldspar” phases (Peretyazhko et al., 2007). The rocks of the inner contact zone from the southwestern flank in general has aphyric texture with scarce (up to 1–2%) phenocrysts of quartz and alkali feldspar in a glassy cryptocrystalline groundmass, with prosopite as one of the rock-forming minerals (from 6 to 26 wt %). The innercontact rocks contain domains consisting of “F–Ca glass”, which further is referred to as the F–Ca phase (Peretyazhko et al., 2007). The traces of the F–Ca phase replaced by dickite or kaolinite with scarce fluorite crystals were found in the groundmass of Ca- and F-rich porphyritic rocks. In terms of chemical composition, the F–Ca phase differs from fluorite in admixture (up to a few percents) of Si, Al, Na, K, and O. Inclusions of the same phase in the same growth zones with melt inclusions were found in quartz. The formation of rocks enriched in F-Ca phase is thought to be related to the immiscibility of silicate and F–Ca melts in the ongonite magma during formation of the rocks of the massif (Peretyazhko and Savina, 2010a, 2010b).
EXPERIMENTAL TECHNIQUE
Three rock varieties of the Ary-Bulak massif representing porphyritic ongonites (sample ARB-28), porphyritic fluorite-bearing ongonites (sample ARB-24), and aphyric rocks of the inner contact facies (ARB-19) were taken as starting material. All samples for experimental study were given by I.S. Peretyazhko as powders and small rock fragments. The detailed description of the samples is given in (Peretyazhko et al., 2007). The content of major components in rock samples is given in Table 1. The rock samples were additionally ground in jasper mortars. Obtained powders were used in two experimental series.
(1) The first experimental series was carried out at 800 and 700°С and 1 kbar. The water content accounted for 10 wt %. Powders of the rock and aliquots of pure water were loaded in platinum capsules, and then capsules were welded shut. The experiments were carried out in a high-pressure hydrothermal apparatus with external heating and cold seal at the Petrology Department of the Lomonosov Moscow State University, Faculty of Geology. The temperature and pressure were maintained with an accuracy of ±10°С and ±100 bar, respectively. The run duration of the experiments was three days at 800°С and seven days at 700°С. According to methodical works described in the monograph (Gramenistkii et al., 2005), such durations are sufficient to reach equilibrium in the fluorine-bearing granite system at studied P-T parameters. Approximate quenching rate is 60°С/min. The oxygen fugacity inside vessel was buffered through the reaction of water with the nickel-based alloy of the vessel (the Ni–NiO buffer).
(2) The second experimental series was carried out at 750°С, 1 kbar, and the elevated oxygen fugacity corresponding to the Mt–Hem buffer. The duration of the experiments in this case was limited by the permeability rate of hydrogen formed during the reaction through the platinum walls of the capsule. The buffer at given P-T conditions maintains constant oxygen fugacity in the system according to the reaction 4Fe3O4 + 2Н2O = 6Fe2O3 + 2Н2↑, as long as both buffer iron phases exist.
The experiments were carried out at the Institute of Experimental Mineralogy (IEM RAS, Chernogolovka) in internally heated pressure vessel (IHPV, SVGD-7). Powders of samples ARB-19 and ARB-24 used as starting materials were loaded together with 10 wt % water in two small (outside diameter of 4 mm, wall thickness of 0.2 mm) platinum capsules and they were welded shut. The small capsules together with the buffer mixture and water were loaded in the intermediate capsule (5 mm across, 0.2 mm thick). The buffer mixture consisted of magnetite and hematite in proportions 1 : 9. The intermediate capsule was also welded shut. To increase the timing of buffer operation, the intermediate capsule was loaded in an external capsule (7 mm across, 0.2 mm thick walls), which was also filled with a buffer mixture and water. The experiments lasted for two days. The quench rate was to 70–100°С/min. Each preparatory stage, prior to and after the experiment, was accompanied by the high-precision weighing of capsule up to 10–5 g.
After completion of the experiments, the phase composition of the buffer mixture was determined by X-ray analysis. In the external capsule, the buffer consisted of 100% magnetite. The intermediate capsule contained 80% magnetite and 20% hematite. Water was present in all capsules.
The chemical composition of samples was determined at the Laboratory of Local Methods of Analysis at the Petrology Department of the Geological Faculty of the Moscow State University using a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Energy 350 energy-dispersive spectrometer (EDS). Dispersion characterizing the detection limit was 0.05 wt % for F and 0.02 wt % for Na, K, Са, Al, and Si. The determination accuracy is ±10 rel. % at contents up to 1 wt %; ±5 rel % at contents from 1 to 5 wt %; ±2 rel % at contents from 5 to 10 wt %.
EXPERIMENTAL RESULTS
The chemical compositions of glasses after experiments based on EDS analyses are given in Table 1. Starting compositions of ongonites used in the experiments are given according to (Peretyazhko et al., 2007). Figure 2 shows the photos of the experimental products and their phase composition.
(1.1) Experimental conditions: buffer Ni–NiO, T = 800°C, P = 1 kbar, and three-day duration. Melting products of porphyritic ongonites from the central facies of the massif (run 28-800) at given experimental parameters consist of aluminosilicate melt, at the quenching of which glass with small amount (1–2%) of quench crystals is formed. The samples contain single grains of ore minerals, which are close to Nb-rich columbite variety.
Sample of run 24-800 consists of aluminosilicate glass (85%), fluorite (10%), and topaz (5%). Fluorite is represented by equant, frequently round crystals reaching 10 μm in size. Topaz forms irregularly shaped elongated, short columnar, or equant grains. Their size varies from 2 to 10 μm.
Sample of run 19-800 consists of glass (55%), fluorite (15%), topaz (15%), and quartz (15%). The amount of the aluminosilicate glass significantly decreases. Fluorite also occurs as equant shaped or round grains up to 10 μm in size. Topaz is represented by small (up to 5 mm) elongated short columnar crystals. Quartz has no characteristic crystallographic shapes. It is represented by round grains of irregular shape up to 50 μm in size.
(1.2) Experimental conditions: buffer Ni–NiO, T = 700°C, Р = 1 kbar, and seven-day duration. With decreasing temperature up to 700°С, melting products of porphyritic ongonites in the central part of the massif (run 28-700) show no significant changes. As in higher temperature experiments, they consist of aluminosilicate glass and quenched phases. Compared to run 28-800, the amount of quenched phases slightly increased up to 5–7%.
Run 24-700 products in addition to aluminosilicate glass, fluorite, and topaz, contains plagioclase. The sample consists of approximately 80% glass, 10% fluorite, 7% topaz, and 3% plagioclase. The size of fluorite grains increases roughly up to 15 μm. The grains have both round and faceted morphology. Plagioclase is represented by up to 20 μm elongated subhedral or skeletal crystals of andesine composition. Topaz (up to 15 μm) frequently shows well expressed zoning. The fluorine to oxygen atomic ratio is approximately 1 : 2 in the core and fluorine content increases to the rim.
Run 19-700 products in addition to glass, quartz, topaz, and fluorite, contains plagioclase, which is represented by up to 10-μm elongated euhedral crystals corresponding to bytownite. Fluorite occurs as equant grains up to 20 μm in size. Topaz forms small (up to 5 μm) grains of irregular shape, which sometimes show zoning. The F/O atomic ratio in the topaz is approximately 1/2. Quartz is represented by large (up to 70 μm) segregations of round irregular shape. Porphyric phenocrysts of the quartz contain inclusions of glass, plagioclase, and topaz.
(2) Experimental conditions: buffer Mt–Hem, T = 750°C, Р = 1 kbar, and two-day duration. An increase of oxygen fugacity in the studied samples is not accompanied by significant changes in phase associations. The phase composition of run products of samples ARB-24 and ARB-19 (runs 24-750 and 19-750, respectively) at given parameters was the same as at melting of these samples at 800°С and oxygen fugacity corresponding to the Ni–NiO buffer. Shape, crystal size, and proportions of the phases also remain constant.
Since round fluorite crystals are interpreted by some researchers as quench products of the fluoride–calcium melt, we considered in detail the morphological features of this phase. Fluorite in experimental samples is represented by both shaped crystals with well-expressed facets and apexes and round grains devoid of crystallographic outlines. It should be noted that in all studied experimental samples, fluorite grains demonstrate a gradual transition from shaped to round morphology. Figure 3a shows sample fragment, which contains fluorite grains with well-expressed apexes and facets (Flu I); grains with expressed facets and smoothed apexes (Flu II); round grains with traces of facets (Flu III), and round grains without facets (Flu IV). Some samples contain grains where only some facet and apexes are well-formed, while others have round outlines (Fig. 3b).
The proportions of well-shaped and round fluorites depend on the starting composition of the system. Sample ARB-24 yielded experimental samples with the predominance of well-shaped fluorite (approximately 80% of total fluorite content) and subordinate round grains. In run products obtained from sample ARB-19, fluorite with well-formed apexes account for 5%, traces of facets occur in 80%, and remained 15% grains are round and lack facets. A change of temperature and oxygen fugacity in experiments does not lead to a change of grain morphology and proportions of well-shaped and round varieties.
Fluorite grains show no characteristic signs of disequilibrium crystallization from an overcooled melt during sample quenching. The samples show no skeletal or edge growth forms. The boundary between the grains and host silicate glass in all studied samples is sharp, clear. The absence of porosity along boundary and within phases suggests against the volatile loss during quenching. In addition, the absence of contraction fractures near fluorite boundary excludes a significant decrease of volume of this phase during quenching.
According to analysis (10–15 measurements in each sample), the Ca and F proportions in obtained fluorite are close to stoichiometric. The faceted and round grains within a single sample show no significant difference in trace element composition. Both round and well-shaped fluorites within single sample have statistically similar contents of oxygen and other trace elements.
It was found that the oxygen admixture significantly increases in fluorite under Mt–Hem buffer conditions (Table 2). Compared to conditions corresponding to the Ni–NiO buffer, the oxygen content increased by approximately 1 wt %. An increase of trace elements in the grains does not affect the described above morphological features. The growth of oxygen content is observed both in round and faceted fluorites.
DISCUSSION
Obtained experimental samples reproduce equilibrium phase associations in the system at given parameters. They do not take into account exchange between a melt and host rocks, as well as other irreversible processes that are possible in natural systems. A change of glass composition in the obtained experimental samples with temperature decrease reflects a compositional change of residual melt in each rock variety during its crystallization. If all rock varieties of the Ary-Bulak massif are the products of crystallization differentiation of a common magmatic melt, a change of their bulk composition (non-volatile components) from the highest- to the lowest temperature varieties should coincide with the compositional evolution of experimental glasses.
According to (Kogarko and Krigman, 1981; Anfilogov et al., 1990, 2005; Gramenitskii et al., 2005), the formation of a salt melt in an equilibrium with aluminosilicate melt is also a peculiar feature of crystallization differentiation. Crystallization of rock-forming minerals is accompanied by the growth of incompatible elements in a residual melt. Up to the attainment of solubility limits of the corresponding phases, the melt accumulates water, fluorine, many trace metals, and others. When solubility limits were exceeded, the aqueous fluid, fluoride phases, and diverse accessory minerals began to exsolve from the melt. Fluoride salt melt is formed as a stable phase in equilibrium with silicate melt. Therefore, the appearance of salt melt, as the formation of the low-density aqueous fluid, cannot be considered separately from crystallization differentiation of magmatic melt. If the rock contains crystallization products of salt melt, its melting at correctly chosen volatile components regime will leads to the repeated appearance of this salt melt in equilibrium with silicate melt.
Phase relations obtained in experimental samples from the Ary-Bulak massif are consistent with previous modeling of haplogranite high-F system (Gramenitskii and Shchekina, 1993, Gramenitskii et al., 2005; Alferyeva et al., 2011, 2018a, 2018b; Shchekina et al., 2013). During equilibrium crystallization, fluorite and topaz are formed as liquidus high-F phases from calcium-bearing high-F granite system at 700–800°С and 1 kbar. Salt F–Ca melt is not formed at these parameters and oxygen fugacity corresponding to the Ni–NiO and Mt–Hem buffers.
It was previously shown that the round shape of fluorine-rich phases is not an indicator feature of the presence of silicate–fluoride liquid immiscibility in highly evolved differentiated granite systems. Round crystals of cryolite, cryolithionite, villiaumite, fluorite, and other minerals were described in experimental samples. Gramenitskii et al. (2005) and Veksler (2004) summarized the results of long-term discussion and criteria, which make it possible to distinguish round crystals from drop-like quenching products of immiscible melts. In our experimental series performed at 700–800°С and 1 kbar, fluorite was a crystalline phase. The discovered phase associations were not produced by liquid silicate–salt immiscibility. This is supported by the following facts.
(1) The presence of all transitional morphologies of fluorite grains from round to well-shaped ones at the preservation of major and trace-element composition suggests that all synthesized fluorite grains represent the same phase. At given experimental parameters, both round and faceted grains have similar composition and the same aggregate state.
The diversity of fluorite morphology in the studied samples could be explained by different sections of 3D crystals with round apexes and well-shaped edges and facets.
(2) Absence of skeletal growth shapes and accumulations of skeletal fluorite crystals.
If fluorite presented in the experimental samples at given parameters was liquid, it would be crystallized from overcooled melt during quenching. The crystallization of the overcooled melt leads to the nucleation of numerous seeds with a weak linear growth of crystals. Drops of fluoride melt at room conditions will be represented by round polycrystalline globules made up of submicron phases (for instance, the globules of aluminofluoride melt in (Alferyeva et al., 2011)).
Fluorite grains in most cases show traces of facets, which unambiguously indicates that they are monocrystals having no signs of disequilibrium crystallization from an overcooled melt.
(3) Preservation of stoichiometric proportions of Ca and F in all experiments regardless of the composition of coexisting silicate melt.
Since the melt is a phase of variable composition, any change of the composition of silicate melt should lead to the corresponding change of salt melt in equilibrium with it. Such a simultaneous change of the composition of equilibrium melts was demonstrated by the example of the silicate and aluminofluoride melts in (Alferyeva et al., 2011). In the experiments considered in this paper, the composition of fluoride phase is preserved close to the fluorite stoichiometry regardless of the composition of equilibrium silicate melt.
In addition to the above-mentioned fact, it should be noted that the obtained fluoride–calcium phase shows no morphological signs of drop merging. This phase does not form separate layers in the upper part of the capsule. Thus, the obtained phase relations cannot be considered as the manifestations of silicate–salt liquid immiscibility.
Data in Table 2 indicate that the high-temperature fluorite forming at the magmatic stage could contain significant amount of admixtures. Since these compositions were also obtained for well-shaped crystals, we can state that the discovered amount of admixtures in fluorite cannot serve as evidence for the existence of F–Ca melt.
An increase of the oxygen content in fluorite could be caused by weak shift to the right-hand side of the equilibrium:
which proceeds owing to the increase of oxygen fugacity in the system. This suggests the possible partial substitution of fluorine for oxygen in the fluorite structure. In this case, obtained sufficiently large content of oxygen in fluorite should affect its unit cell parameters. This conclusion was obtained by (Peretyazhko and Savina, 2020; Peretyazhko et al., 2020).
According to literature data (Holtz et al., 2001), the liquidus temperature of water-saturated granite melt at up to 1 kbar is no more than 800°С, which significantly decreases with fluorine addition in the melt (Kovalenko, 1979; Manning, 1981; Holtz et al., 1993). The crystallization temperatures of ongonites from different massifs of the Transbaikalian region are estimated within 600–700°С (Syritso et al., 2012). As mentioned in (Peretyazhko and Savina, 2010b), numerous xenoliths of quartz–mica schists in the aphyric rocks of the Ary-Bulak massif show no signs of even partial melting. This is inconsistent with extremely high temperature of ongonite melt during emplacement. Based on the complete homogenization temperature of melt inclusions, the estimated liquidus temperature of the Ary-Bulak ongonite melt is within 600–750°С (Antipin et al., 2009; Peretyazhko and Savina, 2010b). According to different sources, the possible fluid pressure range varies from 400–800 (Peretyazhko, 2009) to 1000 bar (Naumov et al., 1990).
Experimental series showed that two rock varieties represented by ARB-19 and ARB-24 samples have a high temperature of complete melting >800°С which is not typically of F-rich rocks. Among the studied samples, the lower temperature was determined only for porphyritic ongonite (sample ARB-28). The rock-forming minerals are not formed yet at 700°С in it. The Ca content in this rock variety is the closest to that of classical ongonites.
The run products of aphyric rocks from the inner contact facies (sample ARB-19) and fluorite-bearing porphyritic ongonites (sample ARB-24) have different liquidus assemblages. In addition to fluorite, topaz, and plagioclase, the stable phase in 19-800, 19-750, and 19-700 run products is quartz. The starting composition of the near-contact facies (Table 1, sample ARB-19) is 10 wt % lower in silica than porphyritic Ca-F ongonite (sample ARB-24). The appearance of liquidus quartz (sample ARB-19) is rather caused by its higher fluorine composition. The elevated fluorine content leads to the widening of the quartz stability field (Kovalenko and Kovalenko, 1976; Kovalenko, 1979; Manning, 1981) and a decrease of silica content in equilibrium melts.
The comparison of data on natural and experimentally obtained samples reveals some disagreements. According to (Peretyazhko et al., 2007; Peretyazhko and Savina, 2010b), the phenocrysts (up to 20 vol %, 1–5 mm) in the porphyritic ongonites (sample ARB-24) are represented by sanidine, albite, quartz, single crystals of topaz and biotite–zinnwaldite; aphyric rocks rarely contain phenocrysts of quartz and sanidine, but sample ARB-19 contains large (up to 1 mm) segregations of prosopite CaAl2F4(OH)4. It was additionally established that interstices between groundmass minerals in both rock types are filled with submicron intergrowths of “fluorite (F–Ca)” (up to 12–13 wt %) and “K-feldspathic” phases, as well as “prosopite” (up to 26 wt %) phase in the aphyric rocks. In the experimentally obtained sample 24-800, the predominant liquidus phase is fluorite, with subordinate topaz, while plagioclase is crystallized at temperature decrease to 700°С (run 24-700). Run products (sample ARB-19) contain approximately equal amounts of fluorite, topaz, and quartz phenocrysts, while plagioclase appears at 700°С. Experimentally obtained plagioclase in run 24-700 sample corresponds to andesine, while that in run 19-700, to bytownite, whereas natural samples contain albite.
In natural samples, F–Ca-rich domains interpreted as the crystallization products of salt F–Ca melt (Peretyazhko and Savina, 2010a, 2010b) ubiquitously contain significant admixture of oxygen, aluminum, and silica, for instance, sample ARB-24 contains 10.2 wt % O and 2.7 wt % Al (Peretyazhko et al., 2007). Fluorite in obtained experimental samples also contains oxygen, but in much lower contents. A change of oxygen fugacity within values corresponding to the Ni–NiO and Mt–Hem buffers leads to a slight increase of oxygen admixture in fluorite and does not facilitate the appearance of the F–Ca melt.
Variation trends of a residual silicate melt with temperature decrease in runs 19-800, 19-700, 24-800, and 24-700 differ from common trends typical of felsic F-bearing rocks. Owing to the low F content in rock-forming minerals, the composition of residual granite melt during crystallization differentiation usually changes toward increasing F content. In the presented experimental series, a temperature decrease is accompanied by a decrease of fluorine content and increase of silica content in the melting products of rocks from the inner contact facies and porphyritic fluorite-bearing ongonite (Fig. 4, Table 1). During crystallization, fluorine is consumed for the formation of fluorite and topaz. A similar systematic decrease of F and Na content in the glass of melt inclusions in quartz and topaz of the Ary-Bulak ongonites caused by the separation of cryolithionite Na3Li3Al2F12 from a residual melt is reported in (Agangi et al., 2014, see Fig. 4).
The analyzed compositions of glasses of runs 28-800 and 28-700 practically do not depend on temperature and are close to the bulk composition of the rock (Figs. 4 and 5).
The bulk composition of rocks from the inner contact zone (sample ARB-19) to fluorite-bearing porphyritic ongonites (sample ARB-24) changes (Fig. 5) toward increasing Si/Al ratio at relatively insignificant change of total alkalis and alkali earth metals. Experimentally obtained trends of samples, in contrast, show a significant decrease of total (Na + K + Ca) in the residual melts (Fig. 5a). From the inner contact rocks to fluorite-bearing ongonites, the Na/K ratio significantly increases. In the residual melts at 800°С, this ratio is approximately equal to the values in the bulk compositions, while at 700°С the melt becomes more potassium (Fig. 5b). According to the experimentally obtained variation trends of the residual melt, samples ARB-19 and ARB-24 are not successive derivatives of a common magma melt.
According to (Peretyazhko et al., 2007; Peretyazhko and Savina, 2010a; 2010b), the formation of marginal zone of Ca- and F-rich porphyritic and aphyric rocks was related to the relatively low-temperature crystallization of immiscible melts of the aluminosilicate and fluoride–calcium compositions and their subsequent metasomatic transformations through the interaction with NaF-bearing aqueous fluids of the P–Q type. However, these works do not describe process that provided the appearance of liquid immiscibility of such type. The formation of the Ary-Bulak massif through the crystallization differentiation does not explain the high Ca content (up to 15 wt %) in the high-F magmatic system.
In most of the studied occurrences of highly evolved granites and their subvolcanic analogues, the calcium content accounts for no more than a tenths of a percent. Even in common biotite granites and rapakivi granites, which are considered to be parental for high-F topaz- and cryolite-bearing granites, the СаО content rarely exceeds 2 wt % (Antipin et al., 1999, 2002, 2006; Vladimirov et al., 2007, 2012; Zaraisky, 2004; Zaraisky et al., 2009; Kuznetsov et al., 2004; Syritso et al., 2001, Syritso, 2002; Badanina et al., 2004; Haapala, 1997; Lenharo et al., 2003; Webster et al., 2004, and others). The classical ongonites of the Ongon–Khaerkhan deposit in Mongolia have extremely low (<0.5 wt %) Ca content (Kovalenko et al., 1971; Kovalenko and Kovalenko, 1976). The mechanism of the joint Ca and F accumulation in a melt at crystallization conditions of granite magma (Т ≤ 800°С) cannot be explained within the crystallization differentiation theory.
The coexistence of Ca and F in a granite melt is limited by the low solubility of fluorite in it. This is confirmed by both presented experimental series and numerous previous experimental works (Price et al., 1999; Scaillet and Macdonald, 2004; Dolejš and Baker, 2004, 2006; Gramenitskii et al., 2005; Lukkari and Holtz, 2007; Peretyazhko and Savina, 2020). According to (Antipin et al., 2009; Agangi et al., 2014), melt inclusions in topaz from ongonites of the Ary-Bulak massif contain daughter cryolithionite crystals, which also indicate that topaz crystallized from the F-rich low-Ca silicate melt.
Disagreement between experimentally obtained and natural phase relations and the high calcium content and liquidus temperature atypical of the high-F melts may indicate that the processes leading to the formation of the entire rock diversity of the massif were disequilibrium and irreversible. The repeated experimental melting of rocks obtained by these irreversible processes will produce new phase associations different from natural ones. Such irreversible processes could be the interaction of highly reactive fluorine-bearing granite melt with host rocks or their xenoliths, sharp disequilibrium changes of P-Т parameters or fluid regime of the system, as well as metasomatic reworking of already crystallized rocks.
We support the idea that Ca-bearing host rocks affect the composition of aphyric rocks of the inner contact facies of the southwestern flank of the massif (sample ARB-19). This idea was proposed by (Antipin et al., 2009) based on the mineralogical-geochemical studies. The unusual composition of the inner contact rocks was explained by the assimilation of limestones of the Ust-Borzya Formation, which hosts the Ary-Bulak massif. Peretyazhko et al. (2011) argue against the assimilation based on the revealed high-Sr varieties of Ca-rich ongonites, the absence of carbonate xenoliths, as well as the low CO2 content in primary fluid inclusions. However, according to experimental data (Gramenitskii et al., 2005), the high-Sr varieties of fluorite-bearing ongonites could be formed by Sr accumulation in fluorite. A mechanism of the interaction of carbonates and fluorine-bearing silicate melts has been poorly studied yet. According to our previous results (Alferyeva et al., 2020b), the intense redistribution of matter at boundary between them and, likely, the high fluorine activity could provide the decarbonatization of calcite already at the magmatic stage.
Therefore, in our opinion, the problem of calcium source in fluorine-rich rocks has not been solved completely and requires further study, including the more accurate determination of the abundance of Ca-rich ongonites. Of great significance was likely the influence of metasomatic or autometasomatic fluids, especially in the inner contact zone of the massif.
Obtained results give grounds to suggest that the porphyritic varieties of fluorite-bearing ongonites (sample ARB-24), as is the case the inner contact rocks (ARB-19), bear traces of interaction with host rocks. This statement is supported by the following experimentally obtained facts: (1) melts that produced porphyritic fluorite-bearing ongonites show the high liquidus temperature (>800°С), as the melts of the inner contact rocks, (2) the composition of phenocrysts in natural samples differ from that of their melting products; (3) plagioclase from experimental samples has the higher calcium composition, (4) diagrams in Figs. 4 and 5 show near parallel variation trends of melts during crystallization differentiation. It is possible that the elevated Ca content in the rock from sample ARB-24, as in the inner contact rock is caused by the assimilation of host limestones.
Experiments on melting/crystallization of definite samples do not reflect all natural processes responsible for the formation of the Ary-Bulak massif. The crystallization differentiation of ongonite melt was likely accompanied by processes that were not taken into account in the proposed experimental series. It is possible that the segregations of the F–Ca phase described in nature (Peretyazhko and Savina, 2010a, 2010b; Peretyazhko and Savina, 2020) were also caused by irreversible processes, which are not reproduced by simple experiments on direct melting of massif rocks.
The presence of liquid immiscibility in the CaF2-granite system was confirmed under experimental conditions at P = 1 atm (Yang and van Hinsberg, 2019) within the temperature range from 1100–1200 to 600–700°C, while melting of fluorite-rich rhyolites at P = 5.5–1 kbar yields F–Ca melt at T = 1250–750°C (Peretyazhko et al., 2018b; Suk et al., 2018; Peretyazhko et al., 2020). We suggest that the determined wide temperature range of liquid immiscibility is related to the erroneous interpretation of crystallized fluorite as a salt F-Ca melt. The experimental studies on melting of rocks of the Ary-Bulak massif under subliquidus conditions at Т = 800–700°С, Р = 1 kbar, water content of 10 wt %, and oxygen fugacity corresponding to the Ni–NiO and Mt-Hem buffers did not reveal the liquid immiscibility of silicate and salt F-Ca melts.
CONCLUSIONS
(1) Obtained experimental samples did not reveal signs of silicate–fluoride liquid immiscibility. At given parameters, F–Ca melt is not formed, while the equilibrium liquidus high-F phases are fluorite and topaz.
(2) A change of oxygen fugacity within the Ni–NiO and Mt–Hem buffers shows no significant effect on the phase relations in the system.
(3) Liquidus temperature of rocks of the inner contact facies and fluorite-bearing ongonites of the Ary-Bulak massif is higher than 800°С, which is not typical of high-F granitoids. Standard liquidus temperature below 700°С was determined only for low-Ca porphyritic ongonites.
(4) The rocks of the Ary-Bulak massif are not successive products of crystallization differentiation of a common magmatic melt. Their formation, in addition to the crystallization differentiation, was contributed by irreversible processes, which were not taken into account in the carried out experimental studies. These processes involve the exchange with host rocks, sharp significant changes of P-T parameters or fluid regime, and others.
Change history
24 July 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S0869591122330013
REFERENCES
Agangi, А., Kamenetsky, V.S., Hofmann, A. et al., Crystallisation of magmatic topaz and implications for Nb–Ta–W mineralisation in F-rich silicic melts — The Ary-Bulak ongonite massif, Litho, 2014, vol. 202–203, pp. 317–330.
Alferyeva, Ya.O., Gramenitskii, E.N., and Shchekina, T.I., Experimental study of phase relations in a lithium-bearing fluorine-rich haplogranite and nepheline syenite system, Geochem. Int., 2011, no. 7, pp. 676–690.
Alferyeva, Ya.O., Novikova, A.S., and Dmitrieva, A.S., Experimental study of phase relations during crystallization of ongonite melt of the Ary-Bulak massif, Tr. Vserossiiskogo ezhegodnogo seminara po eksperimental’noi mineralogii, petrologii i geokhimii (Proc. All-Russian Annual Seminar on Experimental Mineralogy, Petrology, and Geochemistry), (Mosow, 2018a), pp. 93–97.
Alferyeva, Ya.O., Shchekina, T.I., and Gramenitskii, E.N., The limiting contents of fluorine and water in highly differentiated granite melts, Moscow Univ. Geol. Bull., 2018b, vol. 73, no. 3, pp. 390–396.
Alferyeva, Ya.O., Gramenitskii, E.N., and Shchekina, T.I., Changes in the Ta/Nb ratio in successively formed differentiates of granite melt (calculations based on experimental data), Russ. Geol. Geophys., 2020a, vol. 61, no. 1, pp. 26–35.
Alferyeva, Ya.O., Novikova, A.S., and Gramenitskii, E.N., Interaction of fluorine-bearing granite melt as possible reason for the formation of high-calcium ongonites, Tr. Vserossiiskogo ezhegodnogo seminara po eksperimental’noi mineralogii, petrologii i geokhimii (Proc. All-Russian Annual Seminar on Experimental Mineralogy, Petrology, and Geochemistry), Moscow: 2020b, pp. 98–100.
Anfilogov, V.N., Bobylev, I.B., Anfilogova, G.I., and Zyuzeva, N.A., Stroenie i svoistva silikatno-galogenidnykh rasplavov (Structure and Properties of Silicate–Halogenide Melts), Moscow: Nauka, 1990.
Anfilogov, V.N., Bykov, V.N., and Osipov, A.A. Silikatnye rasplavy (Silicate Melts), Moscow: Nauka, 2005.
Antipin, V.S., Savina, E.A., Mitichkin, M.A., and Perelyaev, V.I., Rare-metal lithium–fluorine granites, ongonites, and topazites of the southern Baikal Region, Petrology, 1999, vol. 7, no. 2, pp. 147–159.
Antipin, V.S., Kholls, K., Mitichkin, M.A., et al., Elvans of Cornwall (England) and Southern Siberia as subvolcanic counterparts of subalkalic rare metal granites, Russ. Geol. Geophys., 2002, vol. 43, no. 9, pp. 847–857.
Antipin, V.S., Savina, E.A., and Mitichkin M.A., Geochemistry and formation conditions of rare-metal granites with various fluorine-bearing minerals (fluorite, topaz, and cryolite), Geochem. Int., 2006, no. 10, pp. 965–975.
Antipin, V.S., Andreeva, I.A. Kovalenko, V.I. and Kuznetsov, V.A., Geochemical specifics of ongonites in the Ary-Bulak Massif, Eastern Transbaikalia, Petrology, 2009, vol. 17, no. 6, pp. 601–612.
Badanina, E.V., Veksler, I.V., Thomas, R., et al., Magmatic evolution of Li–F, rare-metal granites: a case study of melt inclusions in the Khangilay complex, eastern Transbaikalia (Russia), Chem. Geol., 2004, no. 210, pp. 113–133.
Badanina, E.V., Syritso, L.F., Volkova, E.V., et al., Composition of Li–F granite melt and its evolution during the formation of the ore-bearing Orlovka Massif in Eastern Transbaikalia, Petrology, 2010, vol. 18, no. 2, pp. 139–167.
Beskin, S.M., Zagorskii, V.E., Kuznetsova, L.G., et al., Etyka rare-metal field in Eastern Transbaikalia (East Siberia), Geol. Rudn. Mestorozhd., 1994, vol. 36, no. 4, pp. 310–325.
Dolejš, D. and Baker, D.R., Thermodynamic analysis of the system Na2O–K2O–CaO–Al2O3–SiO2–H2O–F2O–1: stability of fluorine-bearing minerals in felsic igneous suites, Contrib. Mineral. Petrol., 2004, vol. 146, pp. 762–778.
Dolejš, D. and Baker, D.R., Fluorite solubility in hydrous haplogranitic melts at 100 MPa, Chem. Geol., 2006, vol. 225, pp. 40–60.
Gramenitskii, E.N. and Shchekina, T.I., Phase relations on liquidus of fluorine-bearing system, Geokhimiya, 1993, no. 6, pp. 821–840.
Gramenitskii, E.N., Shchekina, T.I., and Devyatova V.N., Fazovye otnosheniya vo ftorsoderzhashchikh granitnoi i nefelin-sienitovoi sistemakh i raspredelenie elementov mezhdu fazami (Phase relations in fluorine-bearing granitic and nepheline–syenite systems and distribution of elements between phases), Moscow: GEOS, 2005.
Haapala, I., Magmatic and postmagmatic processes in tin-mineralized granites: Topaz-bearing leucogranite in the Eurajoki rapakivi granite stock, Finland, J. Petrol., 1997, vol. 38, no. 12, pp. 1645–1659.
Holtz, F., Dingwell, D.B., and Behrens, H., Effects of F, B2O3 and P2O5 on the solubility of water in haplogranite melts compared to natural silicate melts, Contrib. Mineral. Petrol., 1993, vol. 113, no. 4, pp. 492–501.
Holtz, F., Johannes, W., Tamic, N., and Behrens, H., Maximum and minimum water contents of granitic melts generated in the crust: a reevaluation and implications, Lithos, 2001, vol. 56, no. 1, pp. 1–14.
Kogarko, L.N. and Krigman, L.D., Ftor v silikatnykh rasplavakh i magmakh (Fluorine in Silicate Melts and Magmas), Moscow: Nauka, 1981.
Kostitsyn, Yu.A., Kovalenko, V.I., and Yarmolyuk, V.V., Rb-Sr-isochron dating of the Ary-Bulak ongonite stock (Eastern Transbaikalia), Dokl. Akad. Nauk, 1995, vol. 343, no. 3, pp. 381-384.
Kovalenko, N.I., Eksperimental’noe issledovanie obrazovaniya redkometall’nykh litii-ftoristykh granitov (Experimental Study of the Formation of Rare-Metal Lithium–Fluorine Granites), Moscow: Nauka, 1979.
Kovalenko, V.I. and Kovalenko, N.I., Ongonity - subvulkanicheskie analogi redkometal’nykh litii-ftoristykh granitov (Ongonites as Subvolcanic Analogues of Rare-Metal Lithium–Fluorine Granites), Moscow: Nauka, 1976.
Kovalenko, V.I., Kuz’min, M.I., Antipin, V.S., and Petrov, L.L., Topaz-bearing quartz keratophyre (ongonite) as a new variety of subvolcanic vein magmatic rocks, Dokl. Akad. Nauk SSSR, 1971, vol. 199, no. 2, pp. 430–433.
Kovalenko, V.I., Kostitsyn, Yu.A., Yarmolyuk, V.V., et al., Magma sources and the isotopic (Sr and Nd) evolution of Li–F rare-metal granites, Petrology, 1999, vol. 7, no. 4, pp. 383–409.
Kuznetsov, V.A., Andreeva, I.A., Kovalenko, V.I., et al., Abundance of water and trace elements in the ongonite melt of the Ary-Bulak Massif, Eastern Transbaikal Region: evidence from study of melt inclusions, Dokl. Earth Sci., 2004, vol. 396, no. 4, pp. 571–576.
Lenharo, S.L.R., Pollard, P.J., and Born, H., Petrology and textural evolution of granites associated with tin and rare-metal mineralization at the Pitinga mine, Amazonas, Brazil, Lithos, 2003, vol. 66, pp. 37–61.
Lukkari, S. and Holtz, F., Phase relations of F-enriched peraluminous granite: an experimental study of the Kymi topaz granite stock, southern Finland, Contrib. Mineral. Petrol., 2007, vol. 153, pp. 273–288.
Manning, D.A.C., The effect of fluorine on liquidus phase relationships in the system Qz–Ab–Or with excess water at 1 kb, Contrib. Mineral. Petrol., 1981, vol. 76, pp. 206–215.
Naumov, V.B., Solovova, I.P., Kovalenko, V.I., and Guzhova, A.V., Crystallization of topaz, albite, K-feldspar, mica, and columbite from ongonite melt, Geokhimiya, 1990, no. 8, pp. 1200–1205.
Peretyazhko, I.S., Zagorsky, V.E., Tsareva, E.A., and Sapozhnikov, A.N., Immiscibility of calcium fluoride and aluminosilicate melts in ongonite from the Ary-Bulak Intrusion, Eastern Transbaikal Region, Dokl. Earth Sci., 2007, vol. 413, no. 2, pp. 315–320.
Peretyazhko, I.S., Inclusions of magmatic fluids: P–V–T–x properties of aqueous salt solutions of various types and petrological implications, Petrology, 2009, vol. 17, no. 2, pp. 178–201.
Peretyazhko, I.S. and Savina, E.A., Tetrad effects in the rare earth element patterns of granitoid rocks as an indicator of fluoride–silicate liquid immiscibility in magmatic systems, Petrology, 2010a, vol. 18, no. 5, pp. 514–543.
Peretyazhko, I.S. and Savina, E.A., Fluid and magmatic processes in the formation of the Ary-Bulak ongonite massif (eastern Transbaikalia), Russ. Geol. Geophys., 2010b, vol. 51, no. 10, pp. 1110–1125.
Peretyazhko, I.S. and Savina, E.A., Fluoride–calcium (F–Ca) melt in rhyolitic magma: evidence from fluorite-rich rhyolites of the Nyalga Basin, Central Mongolia, Lithos, 2020, vol. 354–355, pp. 1–25.
Peretyazhko, I.S., Savina, E.A., Dril’, S.I., and Gerasimov, N.S., Rb-Sr isotopic system and Rb and Sr partitioning in the rocks of the Ary-Bulak ongonite massif formed with the participation of fluoride–silicate magmatic immiscibility, Russ. Geol. Geophys., 2011, vol. 52, no. 11, pp. 1401–1411.
Peretyazhko, I.S., Savina, E.A., Karmanov, N.S., and Dmitrieva, A.S., Immiscibility of fluoride–calcium and silicate melts in trachyrhyolitic magma: data on acidic volcanic rocks from the Nyalga Basin, Central Mongolia, Petrology, 2018a, vol. 26, no. 4, pp. 389–413.
Peretyazhko, I.S., Savina, E.A., Kotelnikov, A.R., and Suk, N.I., Partitioning of trace elements between fluoride–calcium and trachyte–rhyolite immiscible melts, Tr. Vserossiiskogo ezhegodnogo seminara po eksperimental’noi mineralogii, petrologii i geokhimii (Proc. All-Russian Annual Seminar on Experimental Mineralogy, Petrology, and Geochemistry), (Moscow, GEOKhI RAN, 2018b), pp. 125–128.
Peretyazhko, I.S., Savina, E.A., Suk, N.I., et al., Evolution of the fluoride–calcium melt composition according to experimental data and fluorite formation in rhyolites, Petrology, 2020, vol. 28, no. 3, pp. 221–245.
Potter, N.J., Kamenetsky, V.S., Simonetti, A., and Goemann, K., Different types of liquid immiscibility in carbonatite magmas: a case study of the Oldoinyo Lengai 1993 lava and melt inclusions, Chem. Geol., 2017, vol. 455, pp. 376–384.
Price, J.D., Hogan, J.P., Gilbert, M.C., et al., Experimental study of titanite-fluorite equilibria in the A-type Mount Scott Granite: implications for assessing F contents of felsic magma, Geology, 1999, vol. 27, no. 10, pp. 951–954.
Scaillet, B. and Macdonald, R., Fluorite stability in silicic magmas, Contrib. Mineral. Petrol., 2004, vol. 147, pp. 319–329.
Shchekina, T.I., Gramenitskii, E.N., and Alferyeva, Ya.O., Leucocratic magmatic melts with the maximum fluorine concentrations: experiment and relations in nature, Petrology, 2013, vol. 21, no. 5, pp. 454–470.
Suk, N.I., Kotel’nikov, A.R., Peretyazhko, I.S., and Savina, E.A., Evolution of trachyrhyolite melts: experimental data, Tr. Vserossiiskogo ezhegodnogo seminara po eksperimental’noi mineralogii, petrologii i geokhimii (Proc. All-Russian Annual Seminar on Experimental Mineralogy, Petrology, and Geochemistry), Moscow: GEOKhI RAS, 2018, pp. 129–132.
Syritso, L.F., Mezozoiskie granitoidy Vostochnogo Zabaikal’ya i problemy redkometal’nogo orudeneniya (Mesozoic Granitoids of Eastern Transbaikalia and Problems of Rare-Metal Mineralization), St. Petersburg: Izd-vo SPbGU, 2002.
Syritso, L.F., Tabuns, E.V., Volkova, E.V., et al., Model for the genesis of Li–F granites in the Orlovka Massif, Eastern Transbaikalia, Petrology, 2001, vol. 9, no. 3, pp. 268–289.
Syritso, L.F., Badanina, E.V., Abushkevich, V.S., et al., Volcanoplutonic association of felsic rocks in the rare-metal ore units of Transbaikalia: geochemistry of rocks and melts, age, and P-T conditions of their crystallization, Petrology, 2012, vol. 20, no. 6, pp. 567–592.
Vasyukova, O. and Williams-Jones, A.E., Fluoride-silicate melt immiscibility and its role in REE ore formation: Evidence from the Strange Lake rare metal deposit, Quebec–Labrador, Canada, Geochim. Cosmochim. Acta, 2014, vol. 139, pp. 110–130.
Veksler, I., Liquid immiscibility and its role at the magmatic–hydrothermal transition: à summary of experimental studies, Chem. Geol., 2004, vol. 210, pp. 7–31.
Vladimirov, A.G., Annikova, I.Yu., and Antipin, V.S., Ongonite–elvan magmatism of South Siberia, Litosfera, 2007, no. 4, pp. 21–40.
Vladimirov, A.G., Phan Luu Anh, Kruk, N.N., et al., Petrology of the tin-bearing granite–leucogranites of the Pia Oak Massif, Northern Vietnam, Petrology, 2012, vol. 20, no. 6, pp. 545–566.
Webster, J.D., Thomas, R., Forster, H., et al., Geochemical evolution of halogen-enriched granite magmas and mineralizing fluids of the Zinnwald tin-tungsten mining district, Erzgebirge, Germany, Miner. Deposita, 2004, vol. 39, pp. 452–472.
Yang, L. and van Hinsberg, V.J., Liquid immiscibility in the CaF2-granite system and trace element partitioning between the immiscible liquids, Chem. Geol., 2019, vol. 511, pp. 28–41.
Zaraisky, G.P., Conditions of formation of rare-metal deposits related to granite magmatism, Smirnovskii sbornik (Smirnov Collection of Papers), Moscow: MGU, 2004, pp. 105–192.
Zaraisky, G.P., Aksyuk, A.M., Devyatova, V.N., et al., The Zr/Hf ratio as a fractionation indicator of rare-metal granites, Petrology, 2009, vol. 17, no. 1, pp. 25–45.
ACKNOWLEDGMENTS
We are grateful to A.R. Kotelnikov, D.Yu. Bychkov, and I.V. Veksler for the discussion of our results. We are also grateful to the reviewers V.S. Antipin and I.S. Peretyazhko, whose critical comments helped us to expand and revise the presented material.
Funding
This study was carried out under the government-financed research project “Regimes of Petrogenesis of the Earth’s Inner Geospheres” as well as government-financed project FMUF-2022-0003 reg. no. 1021051201959-6-1.5.6;1.5.4;1.5.2 of the Institute of Experimental Mineralogy of the Russian Academy of Sciences. Analytical data were obtained at the Laboratory of the Local Methods of the Matter Study (Department of the Petrology and Volcanology, Geological Faculty, Moscow State University) on a “JEOL JXA-8230” electron microscope purchased with funds from the Program of the Development of the Moscow University, as well as at the Institute of Experimental Mineralogy of the Russian Academy of Sciences on a D2 Phaser Bruker X-ray diffractometer and Tescan Vega II XMU digital scanning microscope equipped with EDS INCA Energy 450 with INCA x-sight semiconductor Si(Li) detector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alferyeva, Y.O., Chevychelov, V.Y. & Novikova, A.S. Experimental Study of the Crystallization Conditions of Ongonites of the Ary-Bulak Massif (Eastern Transbaikalia). Petrology 30, 212–225 (2022). https://doi.org/10.1134/S0869591122020011
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0869591122020011