Abstract
The anatomical structure of the auditory system and the presence of several complementary levels of information processing allow a person to very quickly and accurately assess a variety of natural sounds that have minimal differences in frequency, duration, or intensity. At the same time, the features of the analysis of auditory information by the deep structures of the brain have not been fully investigated. In particular, the role of the midbrain in the perception of auditory information has not been fully identified. The basis of this study was the data of analysis of the activity of the midbrain in five people, obtained during intraoperative monitoring during surgery for the removal of tumor of the brainstem. Electric potentials were recorded using a depth electrode installed in the brain aqueduct. The activity of the midbrain associated with the response to simple tones was analyzed. Peaks S1, S2 and S3 were associated with the onset of the sound stimulus, peak E was associated with the end of the sound stimulus. Peaks S1, S2, and S3 most likely reflect the conduction of an uneven impulse along the auditory pathway. Peak E reflects the analysis of auditory information in the midbrain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The anatomical structure of the auditory system and the presence of several complementary levels of information processing [1] allow a person to very quickly and accurately evaluate many natural sounds that have minimal differences in frequency, duration, or intensity [2]. Four complexes of nuclei involved in the transmission of auditory information can be distinguished in the brainstem: auditory nuclei, nuclei of the superior olive, lateral lemniscus, and inferior colliculi. The study of these structures in humans is associated with several difficulties due to their localization in the depths of the brain [3].
In the 1970s, brainstem auditory evoked potentials (BAEP), which are recorded from the surface of the head and reflect the conduction of auditory information from the auditory structures of the brainstem and auditory nerve to the cerebral cortex, found wide use in clinics [4]. Changes in BAEP responses make it possible to accurately determine the level of damage to the auditory system [5–7]. The responses of BAEP components have latencies of up to 10 ms and are distinguished in response to the presentation of a stimulus in the form of rectangular clicks that are presented monaurally [8]. The physical feature of clicks is the absence of ascending and descending fronts, which ensure the gradual involvement of auditory fibers in the process, which have the same characteristic frequency, but different activation thresholds. This leads to the fact that in response to a click, most of the auditory fibers are excited, regardless of their frequency and amplitude characteristics [9]. This, in turn, leads to the fact that an impulse, which passes through the auditory nerve to the central auditory system, can be registered by electrodes located at a distance, on the surface, i.e., far-field potentials are recorded. However, BAEP responses only allow assessing the integrity of information transmission along the fibers of the auditory system., but do not reflect the processing of sound stimuli by nervous structures. This makes it difficult to apply this method to research of cognitive processes in the auditory system [10]. In addition, the generally accepted definition of the sources of generation of components of BAEP is controversial [11]. This is largely due to the fact that initially information about the sources of the recorded signal was obtained by invasive electrophysiological studies on animals [12] or based on changes in the BAEP components in various pathologies of the nervous system [13].
More complete information about the activity of structures located in the depths of the brain can be obtained by recording biopotentials using electrodes implanted directly into the structure under study. In humans, such registration is possible only for medical reasons and, as a rule, is carried out using electrodes designed for deep brain stimulation [14], during intraoperative monitoring (IOM) or postoperative control [15]. IOM is held in patients under anesthesia. In this case, the potentials are recorded from the deep structures of the brain against the background of drug-induced reversible inhibition of cortical activity [16]. This allows to record activity of deep structures without influence of the overlying parts of the brain, including the cerebral cortex. The recording electrode is located in the immediate vicinity of the structure, generating an electric signal, i.e., near-field potentials are registered.
The basis of this study was the data of the analysis of the activity of the human midbrain, obtained during intraoperative monitoring in patients under anesthesia during surgery for the removal of volumetric brain tumors. Electrical potentials were recorded using a deep electrode installed in the cerebral aqueduct. Midbrain activity associated with response to simple tones was analyzed.
METHODOLOGY
The study included five patients (four men, one woman) who underwent surgical treatment of brainstem tumors at the Burdenko Neurosurgery Center (Moscow). Characteristics of patients are presented in Table 1. The inclusion criteria for patients in the study were: no involvement in the midbrain tumor process, complete integrity of the auditory system and cognitive functions before surgery.
During neurosurgical operation, access to the fourth ventricle and tumor removal were performed in all patients. The trajectory of the surgical approach made it possible to visualize the cerebral aqueduct throughout its entire length to the third ventricle. For intraoperative monitoring, a disposable silicone (made of medical plastic compound PM-1/42) round-section electrode with a diameter of 2.7 mm with three axisymmetric ring contact electrodes made of 12Kh18N10T steel was installed in the lumen of the cerebral aqueduct. We used an electrode manufactured by “Neuroelect LLC” (Russia) (Fig. 1). The first two contact electrodes were placed under the superior colliculi (rostral electrode) and inferior colliculi (caudal electrode). The third contact electrode was reference and was located in the lumen of the fourth ventricle. The reference electrode was covered with surgical gauze to prevent direct contact with the ependyma of the fourth ventricle. Intraoperative recording of electrical potentials continued for 15 min during continuous infusion of propofol. The dose of propofol was calculated individually by an anesthesiologist to achieve “burst-suppression” pattern of EEG activity , which alternated every 3–4 s. The dose of propofol is indicated in Table 1.
Scheme of the deep electrode and its position in relation to the structures of the brain. (a) Schematic representation of a deep electrode. (b) Layout of the deep electrode relative to the structures of the brainstem: D1, D2, registering contacts (in the aqueductus cerebri); R, referent; SC, superior colliculi; IC, inferior colliculi; PG, periaqueductal gray matter.
All patients who were implanted with a deep electrode for the purpose of intraoperative monitoring had an uncomplicated postoperative period without an increase in cerebral and focal, including midbrain, symptoms. Each patient in the postoperative period underwent neuropsychological testing, which did not reveal any deviations from the preoperative level in the cognitive sphere. All patients showed no signs of hearing loss in the postoperative period.
Registration of evoked potentials (EPs). The sound sequence consisted of simple sinusoidal tones of four types: frequency 600 Hz for 80 ms; 800 Hz for 90 ms; 1000 Hz for 100 ms; and 2000 Hz for 100 ms. In total, there were 100 stimuli in the sequence: 25 stimuli of each type. All sounds had an ascending phase, a plateau, and a descending phase. The interstimulus interval varied from 1100 to 1170 ms. Stimuli were presented binaurally via on-ear headphones.
The sound sequence was presented using the “Presentation” program (Neurobehavioral Systems Inc., USA).
EPs were recorded using a Neuron-Spectrum 4/P (Neurosoft, Russia). Sampling frequency 5000 Hz; high-pass filter (HPF) 0.5 and low-pass filter (LPF) 500 Hz, and notch filter 50 Hz.
During the study, synchronously with the registration of electric potentials from a deep electrode, registration was carried out from needle electrodes located on the surface of the head according to the 10–20% scheme. The number of electrodes varied from 13 to 19 (Table 1). The electrodes that fell into the area of work of the neurosurgeon or were in close proximity to it were excluded from the scheme. A combined ear electrode was used as a indifferent electrode for the electrodes located on the surface of the head. Deep potentials were recorded bipolar with their own referent. The ground electrode, common for deep electrodes and electrodes located on the surface of the head, was placed in the projection of the shoulder joint.
A separate channel recorded an electrogram of sound stimuli, reflecting the current supplied to the headphones during sound stimulation.
Data analysis. The registered potentials were analyzed with “Brainstorm” program [17]. Artifact-free sections of the recording were taken for analysis, performed by the following digital filters: HPF 2 Hz and notch filter 50 Hz.
To improve the accuracy of synchronization of brain responses with the applied stimulus, individual brain responses recorded on deep electrodes were analyzed before EP averaging. To do this, within 5 ms after the onset of sound stimulus, a complex of two peaks was distinguished. These peaks were named after the V and VI BAEP peaks, which reflect the conduction of a nerve impulse through the auditory structures of the midbrain and are reliably distinguished by visual analysis. At the top of the peak V, a mark was placed, relative to which the EP was then averaged in response to a tone of a certain frequency.
Due to the presence of a technical drift (desynchronization) of the event marker relative to the real beginning of the stimulus, the exact beginning of the sound stimulus was identified based on both the responses from the midbrain and the electrogram. This procedure was performed in each individual patient for single responses to the stimulus. To do this, at the first stage, peak V latency was taken as the zero point. Next, the beginning of the stimulus was verified by the electrogram. The reliability criterion was a statistically significant equality of the time intervals from the peak V to the beginning of the stimulus in the electrogram (Fig. 2). The end of the sound stimulus was identified in a similar way. At the final stage, the EP was averaged relative to the peak V.
EPs included a pre-stimulus interval of 100 ms and a post-stimulus interval of 300 ms. EPs for each tone of different frequencies were analyzed separately. We analyzed EPs recorded from both deep electrodes, as well as from two electrodes located on the surface of the head (C3, C4).
Pseudo-EP was calculated as a control. To do this, 25 marks were randomly placed on recordings sections without sound stimuli. Averaging was carried out with respect to these marks.
Statistical analysis was carried out in STATISTICS 10.
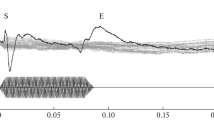
Peak V, peak VI, immediately following peak V, peaks S1, S2, S3, and peak E, following the end of the sound stimulus, were analyzed on the obtained EP responses. Peak names were given based on their perceived functional significance. Peaks S1, S2, and S3, registered immediately after the beginning, are presumably associated with an assessment of the start of the sound, while the E peak is recorded after the end of the sounding of the tone and it is presumably a marker of the end of the sounding of the tone.
The latencies of all components were calculated relative to the peak V. The obtained values were compared in pairs between tones of different frequencies and durations. Statistical analysis of the data included the non-parametric Mann-Whitney test, as well as an assessment of the median values and interquartile ranges of the obtained distributions. These statistical criteria were chosen after testing for the normality of the obtained distributions.
RESULTS
On the EEG from the surface of the head “burst-suppression” activity was recorded. Bursts of electrophysiological activity had the highest amplitude in the frontal electrodes and consisted mainly of oscillations in the α-range. The recordings of electric potentials recorded from deep electrodes did not show “burst-suppression” patterns.
On EP recorded from the surface of the head, unstable peaks were identified that recur in response to a stimulus of the same frequency (Fig. 3). Peaks V, VI, S1, S2, S3, and E were distinguished on the EP responses recorded from deep electrodes. At the same time, the EP peaks recorded from the rostral and caudal deep electrodes differed.
Peaks V, VI, S1, S2, S3, and E were identified from the rostral electrode. Only peaks V, VI and E were clearly distinguished from the caudal electrode, while peaks S1, S2 and S3 had a lower amplitude and unstable morphology, which made it difficult to identify them reliably (Fig. 4).
The latency of peak VI recorded from the rostral and caudal electrodes did not differ from each other. Also, there were no statistically significant differences in this peak in response to stimuli of different frequencies (p > 0.05).
The latencies of peaks S1, S2 and S3 recorded from the rostral electrode did not differ statistically upon presentation of stimuli of different frequencies. The interquartile interval (IQI) of the latencies of peak S3 exceeded that of peaks S1 and S2. The IQI of the latencies of peak S3 recorded in response to 1000 and 2000 Hz stimuli exceeded those for peaks S1 and S2 by a factor of 2 or more (Table 2).
The E peak was identified from both the rostral and caudal electrodes (Fig. 4). IQI of the latency of peak E from the caudal electrode exceeded those from the rostral one (Table 3). Peak E latencies between responses to sound stimuli of different durations significantly differed (p < 0.05) (Fig. 5). When comparing the latencies of peak E recorded in response to stimuli of the same duration but different frequencies no statistically significant differences were found.
DISCUSSION
The basis of the study was data from the analysis of the electrical activity of the midbrain in response to the presentation of simple tones of various frequencies and durations. Registration was carried out as part of routine intraoperative monitoring. This approach is original, since presenting clicks, which are traditionally used in the analysis of the integrity of the auditory system, allows to assess the conduction of a sound signal through the brainstem structures but does not provide information about sound processing [11].
As a result of the study, peaks of EP responses were recorded. Peaks V, VI, S1, S2, and S3 were associated with the beginning of sound stimulus, Peak E was associated with the end of sound stimulus. The proximity of peaks VI, S1, S2, and S3 to peak V in terms of generation time and peak shape suggests that they reflect similar physiological processes. Peak V is located close to the beginning of the sound signal in terms of the time of occurrence. It is at the top of a low-frequency and high-amplitude wave, and peak VI is on the downward slope of this wave. The time interval between peaks V and VI is 1.8 ms. This value is close to the time interval between peaks V and VI of BAEP, which is 1.6 ms [4]. This testifies in favor of the fact that the peaks V and VI recorded at deep electrodes are equivalent to the peaks V and VI of BAEP. Temporal differences between the peaks recorded on the deep electrode and BAEP peaks constitute 12.5%. These differences may reflect the specifics of information transmission through the auditory system under anesthesia. Also, it cannot be ruled out that the time of conduction was affected by the presence of a pathological process in the brainstem and the physical impact on the brain during neurosurgical operation. This suggests that peaks V and VI, and peaks S1, S2, and S3 immediately following them, are associated with the response of the midbrain to the onset of the sound signal.
Peak E differs greatly in generation time and morphology from the other peaks detected on deep electrodes. Its latency, counted from the peak V, differs statistically significantly between tones with different durations and has similar values for tones of the same duration and different frequencies. This allows us to assume that the structures, which generate the peak E of auditory EPs recorded at deep electrodes, respond to the end of the sound signal. Therefore, it can be hypothesized that the generation of the peak E is associated with the end of the presentation of the sound stimulus.
If we assume that peaks S1, S2, S3, and peak E are generated in the cerebral cortex or thalamus, then far-field potentials should be recorded on the deep electrode and, therefore, they should be equally well visualized on signals recorded on both the rostral and caudal electrodes. However, the S1, S2, and S3 components are visible only when the response is recorded on the rostral electrode. In addition, IQI of latencies of the peak E recorded on the caudal electrode, exceed those from the rostral electrode. All these facts indicate that peaks S1, S2, S3, and E are generated by the structure in close proximity to the rostral electrode, i.e., in the midbrain.
If we assume the cortical origin of these peaks, then the cerebral cortex should have a sufficient influx of sensory information and have the necessary level of activity to process this information. However, the “burst–suppression” phenomenon recorded at the moment of stimulation from electrodes located on the surface of the head, and the predominance of α-range activity in the frontal regions during bursts, as well as the absence of pronounced EP responses in response to stimuli, suggest the passage of most of the nervous impulses arising in the process of response to a sound stimulus, along the fibers of the excitatory thalamocortical loop [18]. This type of feedback between the cortex and thalamus, which occurs in a state of anesthesia caused by the action of propofol, indicates an increase in the inhibition of thalamocortical connections by the reticular nucleus of the thalamus and, as a result, the disconnection of the thalamus and cortex [19]. Thus, sensory flow to the cortex is greatly reduced. In addition, the absence of stable EP responses from electrodes located on the surface of the head in response to stimuli indicates a lack of cortical activation, as well as a lack of sensory input into the cortex for the implementation of minimal cognitive processes [20].
This study has several limitations. First of all, this is the impossibility of recording midbrain activity in healthy subjects. In this regard, the emphasis was on a detailed analysis of the data obtained, and not on comparing them with the control group. In addition, the expediency of such registration is limited by the clinical need for intraoperative monitoring, which makes it impossible to recruit large groups of patients. It also imposes restrictions on the duration of research and the impossibility of using longer experimental tasks.
Registration of electrical potentials in a neurosurgical operating room limits the number of electrodes placed on the surface of the head the number of which varied. Structural features of the human brainstem and surgery approach (the entrance to the fourth ventricle and, consequently, to the cerebral aqueduct was carried out through a large occipital cistern) made it difficult to accurately visually assess the location of the deep ring electrodes, so it was not possible to calculate their exact coordinates for further mathematical analysis.
The technical limitations of the study can also be attributed to the features of the presented stimuli. Simple tones had rising and falling fronts, which made it difficult to estimate the position of the beginning and end of the sound in time. In particular, it was not possible to estimate the hearing threshold for midbrain structures in each individual patient. In addition, the error of the method for measuring the beginning and end of the audio signal was up to 2 ms. All this made it unacceptable to count the EP latencies in the traditional way, from the beginning of the audio signal. In this regard, it was decided to consider the latencies of the EP components from the deep component most clearly distinguished on the native EEG recording, the peak V of the rostral ring electrode.
The position of the ground electrode must also be referred to technical limitations. Usually, when registering auditory EPs, the ground electrode is located in the front-sagittal position (FZ or FpZ). Modern digital devices recalculate the signal on all electrodes located on the surface of the head, taking into account the biopotentials of the brain that accumulate on the ground electrode. However, for bipolar channels, which are usually used for control recording of myo- and cardiograms, there is no recalculation described above due to the lack of need for an accurate assessment of the amplitudes of the received signals. The position of the ground electrode in the projection of the shoulder joint reduced but did not completely eliminate the noise on it.
CONCLUSIONS
In this study we analyzed the responses of cerebral EPs recorded from deep electrodes located in the midbrain. The responses to stimuli, which were simple tones, were analyzed. Peaks associated with the beginning—S1, S2, and S3—and the end of the sound stimulus—peak E—were identified. Peaks S1, S2, and S3 most likely reflect the conduction of a nerve impulse along the auditory pathway. Peak E reflects the analysis of sound information in the midbrain.
Change history
08 September 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S0362119722440025
REFERENCES
Principles of Neural Science, Kandel, R., Schwartz, H., Jessell, M., , Eds., 5th ed. New York: McGraw-Hill, 2012, p. 682.
Koroleva, I.V., Kokhlearnaya implantatsiya glukhikh detei i vzroslykh (elektronnoe protezirovanie slukha) (Cochlear Implantation for Deaf Children and Adults: Electronic Prosthetics of Hearing), St. Petersburg: Karo, 2012, p. 33.
Sitek, K., Gulban, O., Calabrese, E., et al., Mapping the human subcortical auditory system using histology, postmortem MRI and in vivo MRI at 7T, eLife, 2019, vol. 8, p. e48932.
Picton, T., Hillyard, S., Krausz, H., and Galambos, R., Human auditory evoked potentials. I: evaluation of components, Electroencephalogr. Clin. Neurophysiol., 1974, vol. 36, no. 2, p. 179.
Nodar, R.H., Hahn, J., and Levine, H.L., Brain stem auditory evoked potentials in determining site of lesion of brain stem gliomas in children, Laryngoscope, 1980, vol. 90, no. 2, p. 258.
Goldie, W.D., van Eyes, J., and Baram, T.Z., Brain stem auditory evoked potentials as a tool in the clinical assessment of children with posterior fossa tumors, J. Child Neurol., 1987, vol. 2, no. 4, p. 272.
Kjaer, M., Localizing brain stem lesions with brain stem auditory evoked potentials, Acta Neurol. Scand., 1980, vol. 61, no. 5, p. 265.
Gnezditskii, V.V., Vyzvannye potentsialy mozga v klinicheskoi praktike (Evoked Brain Potentials in Clinical Practice), Taganrog: Taganrog. Gos. Univ., 1997, p. 65.
Evans, E., The frequency response and other properties of single fibers in the guinea-pig cochlear nerve, J. Physiol., 1972, vol. 226, no. 1, p. 263.
Eggermont, J., Auditory brainstem response, Handb. Clin. Neurol., 2019, vol. 160, p. 451.
Shaw, N., The temporal relationship between the brainstem and primary cortical auditory evoked potentials, Prog. Neurobiol., 1995, vol. 47, no. 2, p. 95.
Møller, A., Jannetta, P., Bennett, M., and Møller, M., Intracranially recorded responses from the human auditory nerve: new insights into the origin of brainstem evoked potentials (BSEPs), Electroencephalogr. Clin. Neurophysiol., 21981, vol. 52, no. 1, p. 18.
Hashimoto, I., Ishiyama, Y., Yoshimoto, T., and Nemoto, S., Brain-stem auditory-evoked potentials recorded directly from human brain-stem and thalamus, Brain, 1981, vol. 104, no. 4, p. 841.
Swann, N., de Hemptinne, C., Miocinovic, S., et al., Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson’s disease, J. Neurosurg., 2018, vol. 128, no. 2, p. 605.
Oknina, L., Kantserova, A., Masherov, E., et al., Functional connectivity between the midbrain and cortex during consciousness recovery after general anesthesia, World J. Pharm. Med. Res., 2019, vol. 5, no. 2, p. 237.
Sahinovic, M.M., Struys, M.M.R.F., and Absalom, A.R., Clinical pharmacokinetics and pharmacodynamics of propofol, Clin. Pharmacokinet., 2018, vol. 57, no. 12, p. 1539.
Tadel, F., Baillet, S., Mosher, J.C., et al., Brainstorm: a user-friendly application for MEG/EEG analysis, Comput. Intell. Neurosci., 2011, vol., 2011, p. 879716.
Ching, S., Cimenser, A., Purdon, P., et al., Thalamocortical model for a propofol-induced α-rhythm associated with loss of consciousness, Proc. Natl. Acad. Sci. U.S.A., 2010, vol. 107, no. 52, p. 22665.
Purdon, P., Sampson, A., Pavone, K., and Brown, E., Clinical electroencephalography for anesthesiologists. Part I: Background and basic signatures, Anesthesiology, 2015, vol. 123, no. 4, p. 937.
Kletecka, J., Holeckova, I., Brenkus, P., et al., Propofol versus sevoflurane anaesthesia: effect on cognitive decline and event-related potentials, J. Clin. Monit. Comput., 2019, vol. 33, no. 4, p. 665.
Funding
The study was funded by the state budget under the state order for 2019–2021 (AAAA-A17-117092040004-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All studies were conducted in accordance with the principles of biomedical ethics formulated in the 1964 Declaration of Helsinki and its subsequent updates. The studies were carried out as part of planned scientific topics and a comprehensive clinical examination in the provision of medical care in Burdenko Neurosurgery Center (Moscow). The study was approved by the local ethics committee.
CONFLICT OF INTERESTS
The authors declare that they do not have a conflict of interests.
INFORMED CONSENT
Each participant in the study provided a voluntary written informed consent signed by the participant after explaining to them the potential risks and benefits, as well as the nature of the upcoming study.
Additional information
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kantserova, A.O., Oknina, L.B., Pitskhelauri, D.I. et al. Evoked Potentials of the Midbrain Associated with the Beginning and End of a Sound of a Simple Tone. Hum Physiol 48, 229–236 (2022). https://doi.org/10.1134/S0362119722030082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722030082