Abstract
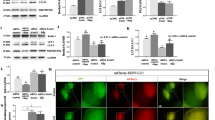
Introduction: Alzheimer’s disease is a chronic neurodegenerative disease leading to neuropsychiatric disorders and cognitive decline. A number of studies demonstrate an important role of the mitogen-activated protein kinase (MAPK) pathway and NLRP3 inflamasomes in β-amyloid metabolism impairment and insulin resistance in Alzheimer’s disease. Objective: To study the expression of NLRP3 in the cells of neuronal and glial nature, as well as the expression of MAPK in the amygdala neurons in animals with experimental Alzheimer’s disease. Materials and methods: Subjects of the study: (1) CD1 mice (males, 4 months old) divided in 2 groups, the experimental group (intra-hippocampal exposure to β-amyloid) and the control group (sham-operated animals); (2) mice with a genetic model of Alzheimer’s disease, the B6SLJ-line Tg (APPSwFlLon, PSEN1*M146L*L286V) 6799Vas (males, 12 months old) and the corresponding control group, C57BL/6xSJL mice (males, 12 months old). Immunohistochemistry on free-floating sections was applied for the study of NLRP3 and MAPK expression in the brain amygdala. Results: We revealed the statistically significant increase in the number of NeuN/NLRP3-positive cells (p = 0.043) of the amygdala in animals with a genetic model of Alzheimer’s disease (29.05 ± 2.67) compared with the control group of animals (17.10 ± 1.95). A same trend was obvious in β-amyloid-induced neurodegeneration (p = 0.021). Intra-hippocampal exposure to β-amyloid caused the decrease of MAPK expression in the amygdala neurons (5.97 ± 0.66) compared with the sham-operated animals (13.25 ± 2.65) (p = 0.018). This was also seen in animals with a genetic model of the Alzheimer’s disease (p = 0.031). Conclusions: The increase of NLRP3 inflammasomes expression in animals with experimental Alzheimer’s disease was found in neurons, but not in astrocytes, along with a decrease of the MAPK expression in neurons of the amygdala. These findings indicate coupling of the inflammatory process and the disorganization on the insulin-signaling mechanisms of the brain during neurodegeneration.



Similar content being viewed by others
REFERENCES
Pierce, A.L., Bullain, S.S., and Kawas, C.H., Late-onset Alzheimer disease, Neurol. Clin., 2017, vol. 35, pp. 283–293. PMID 28410660. doi 10.1016/j.ncl. 2017.01.006
Fessel, J., Amyloid is essential but insufficient for Alzheimer causation: addition of subcellular cofactors is required for dementia, Int. J. Geriatr. Psychiatry, 2017. PMID 28509380. doi 10.1002/gps.4730
Gao, Y., Tan, L., Yu, J.T., and Tan, L., Tau in Alzheimer’s disease: Mechanisms and therapeutic strategies, Curr. Alzheimer Res., 2017. PMID 28413986. doi 10.2174/1567205014666170417111859
Giri, M., Zhang, M., and Lü, Y., Genes associated with Alzheimer’s disease: an overview and current status, Clin. Interv. Aging, 2016, vol. 11, pp. 665–681. PMID 27274215. doi 10.2147/CIA.S105769
Waite, L.M., Treatment for Alzheimer’s disease: has anything changed? Aust Prescriber, 2015, vol. 38, pp. 60–63. PMID 26648618
Banks, W.A., Owen, J.B., and Erickson, M.A., Insulin in the brain: there and back again, Pharmacol. Ther., 2012, vol. 136, pp. 82–93. PMID 22820012. doi 10.1016/j.pharmthera.2012.07.006
King, G.L., Park, K., and Li, Q., Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award Lecture, Diabetes, 2016, vol. 65, pp. 1462–1471. PMID 27222390. doi 10.2337/db16-0152
Chen, Y., Deng, Y., Zhang, B., and Gong, C.X., Deregulation of brain insulin signaling in Alzheimer’s disease, Neurosci. Bull., 2014, vol. 30, pp. 282–294. PMID 24652456. doi 10.1007/s12264-013-1408-x
Ghasemi, R., Dargahi, L., Haeri, A., et al., Brain insulin dysregulation: implication for neurological and neuropsychiatric disorders, Mol. Neurobiol., 2013, vol. 47, pp. 1045–1065. PMID 23335160. doi 10.1007/s12035-013-8404-z
Tong, L., Balazs, R., Thornton, P.L., and Cotman, C.W., β-Amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons, J. Neurosci., 2004, vol. 24, pp. 6799–6809. PMID 15282285. doi 10.1523/ JNEUROSCI.5463-03.2004
Tan, M.S., Yu, J.T., Jiang, T., et al., The NLRP3 inflammasome in Alzheimer’s disease, Mol. Neurobiol., 2013, vol. 48, pp. 875–882. PMID 23686772. doi 10.1007/s12035-013-8475-x
Wen, H., Gris, D., Lei, Y., et al., Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling, Nat. Immunol., 2011, vol. 12, pp. 408–415. PMID 21478880. doi 10.1038/ni.2022
Komleva, Yu.A., Malinovskaya, N.A., Gorina, Ya.V., et al., Expression of CD38 and CD157 molecules in olfactory bulbs of the brain in experimental Alzheimer’s disease, Sib. Med. Obozr., 2015, no. 5, pp. 45–49.
Encinas, J.M. and Enikolopov, G., Identifying and quantitating neural stem and progenitor cells in the adult brain, Methods Cell Biol., 2008, vol. 85, pp. 243–272. PMID 18155466. doi 10.1016/s0091-679x(08)85011-x
Prinz, M., Priller, J., Sisodia, S.S., and Ransohoff, R.M., Heterogeneity of CNS myeloid cells and their roles in neurodegeneration, Nat. Neurosci., 2011, vol. 14, pp. 1227–1235. PMID 21952260. doi 10.1038/nn.2923
Brough, D. and Denes, A., Interleukin-1α and brain inflammation, IUBMB Life, 2015, vol. 67, pp. 323–330. PMID 25906979. doi 10.1002/iub.1377
Dinarello, C.A., Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process, Am. J. Clin. Nutr., 2006, vol. 83, pp. 447S–455S. PMID 16470011
Heneka, M.T., Kummer, M.P., Stutz, A., et al., NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice, Nature, 2013, vol. 493, pp. 674–678. PMID 23254930. doi 10.1038/nature11729
Kaushal, V., Dye, R., Pakavathkumar, P., et al., Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation, Cell Death Differ., 2015, vol. 22, pp. 1676–1686. PMID 25744023. doi 10.1038/ cdd.2015.16
Bergsbaken, T., Fink, S.L., and Cookson, B.T., Pyroptosis: host cell death and inflammation, Nat. Rev. Microbiol., 2009, vol. 7, pp. 99–109.
Tan, M.S., Tan, L., Jiang, T., et al., Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease, Cell Death Dis., 2014, vol. 5, p. e1382. PMID 19148178. doi 10.1038/nrmicro2070
Johann, S., Heitzer, M., Kanagaratnam, M., et al., NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients, Glia, 2015, vol. 63, pp. 2260–2273. PMID 26200799. doi 10.1002/glia.22891
Lau, L.T. and Yu, A.C., Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury, J. Neurotrauma, 2001, vol. 18, pp. 351–359. PMID 11284554. doi 10.1089/ 08977150151071035
Liu, L. and Chan, C., IPAF inflammasome is involved in interleukin-1beta production from astrocytes, induced by palmitate; implications for Alzheimer’s disease, Neurobiol. Aging, 2014, vol. 35, pp. 309–321. PMID 24054992. doi 10.1016/j.neurobiolaging.2013.08.016
Hass, D.T. and Barnstable, C.J., Uncoupling protein 2 in the glial response to stress: implications for neuroprotection, Neural Regen. Res., 2016, vol. 11, pp. 1197–1200. PMID 27651753. doi 10.4103/1673-5374.189159
Kothari, V., Luo, Y., Tornabene, T., et al., High fat diet induces brain insulin resistance and cognitive impairment in mice, Biochim. Biophys. Acta, 2017, vol. 1863, pp. 499–508. PMID 27771511. doi 10.1016/j.bbadis.2016.10.006
Schrijvers, E.M., Witteman, J.C., Sijbrands, E.J., et al., Insulin metabolism and the risk of Alzheimer disease: the Rotterdam study, Neurology, 2010, vol. 75, pp. 1982–1987. PMID 21115952. doi 10.1212/WNL. 0b013e3181ffe4f6
Macesic, M., Lalic, N.M., Kostic, V.S., et al., Impaired insulin sensitivity and secretion in patients with Alzheimer’s disease: the relationship with other atherosclerosis risk factors, Curr. Vasc. Pharmacol., 2017, vol. 15, pp. 158–166. PMID 27599805. doi 10.2174/1570161114666160905170644
Kandimalla, R., Thirumala, V., and Reddy, P.H., Is Alzheimer’s disease a type 3 diabetes? A critical appraisal, Biochim. Biophys. Acta, 2017, vol. 1863, pp. 1078–1089. PMID 27567931. doi 10.1016/j.bbadis.2016.08.018
Cusi, K., Maezono, K., Osman, A., et al., Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle, J. Clin. Invest., 2000, vol. 105, pp. 311–320. PMID 10675357
Gyurkó, M.D., Steták, A., Sőti, C., and Csermely, P., Multitarget network strategies to influence memory and forgetting: the Ras/MAPK pathway as a novel option, Mini-Rev. Med. Chem., 2015, vol. 15, pp. 696–704. PMID 25694072
Moghbelinejad, S., Nassiri-Asl, M., Farivar, T.N., et al., Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats, Toxicol. Lett., 2014, vol. 224, pp. 108–113. PMID 24148604. doi 10.1016/j.toxlet.2013.10.010
Zeng, Y., Zhang, L., and Hu, Z., Cerebral insulin, insulin signaling pathway, and brain angiogenesis, Neurol. Sci., 2016, vol. 37, pp. 9–16. PMID 26442674. doi 10.1007/s10072-015-2386-8
ACKNOWLEDGMENTS
The study was funded by the grant of the President of the Russian Federation given to Russian Leading Research Teams (NSh10241.2016.7).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Gorina, Y.V., Lopatina, O.L., Komleva, Y.K. et al. Expression of MAPK and Inflammasomes in Brain Cells in Experimental Alzheimer’s Disease. Hum Physiol 44, 906–911 (2018). https://doi.org/10.1134/S0362119718080042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119718080042