Abstract
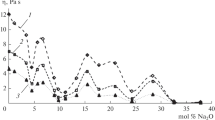
The viscosities (η) of the sodium–borate melts containing 1 wt % mechanically activated lanthanide oxide (Sm2O3, Eu2O3, Er2O3, Tm2O3) are determined with an oscillatory viscometry in the temperature range 950–1650 K. The laws of changing the viscosity as a function of temperature and composition are found, and the temperatures of the onset of solidification of the melts are determined. The dependence of the logarithm of viscosity on the reciprocal temperature is found to have high- and low-temperature sections with different activation energies of viscous flow. The changes in the viscosity are explained in terms of changes in the melt structure.


Similar content being viewed by others
REFERENCES
N. Ye, Y. Zhang, W. Chen, et al., “Growth of nonlinear optical crystal Y0.57La0.72Sc2.71(BO3)4,” J. Cryst. Growth. 292 (2), 464–467 (2006).
T. N. Svetlyakova, N. G. Kononova, A. E. Koch, et al., “Phase formation in the BaB2O4–NaBO2–MBO3 (M = Sc, La, Y) system and the new orthoborate ScBaNa(BO3)2,” Zh. Neorg. Khim. 56 (1), 117–121 (2011).
W. Li, L. Huang, G. Zhang, et al., “Growth and characterization of nonlinear optical crystal Lu0.66La0.95Sc2.39(BO3)4,” J. Cryst. Growth 307 (2), 405–409 (2007).
M. V. Fedorova, N. G. Kononova, A. E. Koch, et al., “Growth of MBo3 (M = La, Y, Sc) and LaSc3(BO3)4 crystals from molten LiBO2–LiF solutions,” Neorg. Mater. 49 (5), 505–510 (2013).
V. V. Rudenko, “Growth of MBO3 (M = In, Lu, Sc) crystals from a solution–melt of the B2O3–PbO–PbF2 system,” Neorg. Mater. 34 (12), 1483–1485 (1998).
M. Masayuki, Y. Hiromasa, and O. Osamu, “Production of silicon-containing GaAs single crystal,” Japan Patent 649898, 1989.
J. D. Mackenzie, “The viscosity, molar volume and electrical conductivity of liquid boron trioxide,” J. Phys. Chem. 52 (11), 1564–1568 (1956).
A. Napolitano, “Viscosity and density of boron trioxide,” J. Amer. Ceram. Soc. 48 (12), 613–616 (1965).
R. A. Eppler, “Viscosity of molten B2O3,” J. Amer. Ceram. Soc. 49 (12), 679–680 (1966).
V. I. Musikhin, E. A. Pastukhov, V. M. Denisov, et al., “Viscosity of melts in the systems based on boron oxide,” Rasplavy, No. 3, 40–45 (1992).
E. A. Pastukhov, S. A. Istomin, A. A. Khokhryakov, et al., “Effect of samarium, terbium, and dysprosium oxides on the physicochemical properties of boron oxide,” Rasplavy, No. 3, 52–57 (1996).
S. A. Istomin, V. V. Ryabov, E. A. Pastukhov, et al., “Effect of mechanochemical treatment of initial mixtures on the physicochemical properties of borosilicate melts,” Rasplavy, No. 3, 3–9 (2008).
S. A. Istomin, A. V. Ivanov, V. V. Ryabov, et al., “Effect of mechanical activation of REM oxides on the viscosity of borate melts,” Rasplavy, No. 4, 11–16 (2011).
S. A. Istomin, A. A. Khokhryakov, V. V. Ryabov, et al., “Effect of mechanically activated REE oxides of the lanthanide group on the viscosity of borate melts,” Rasplavy, No. 5, 69–77 (2014).
V. V. Ryabov, S. A. Istomin, A. A. Khokhryakov, et al., “Viscosity of sodium–borate melts containing mechanically activated additives of REE oxides,” Rasplavy, No. 2, 35–39 (2015).
A. A. Khokhryakov, A. O. Vershinin, A. S. Paivin, et al., “Electronic spectra of molten xNa2O–(100 – x)B2O3–Re2O3 (Re = Sm, Eu) mixtures,” Rasplavy, No. 6, 538–549 (2017).
A. A. Osipov, L. M. Osipova, and V. N. Bykov, Spectroscopy and Structure of Alkali Borate Glasses and Melts (UrO RAN, Yekaterinburg, 2009).
K. Mitsury, M. Yu, and K. Seiji, “Temperature dependence of elastic properties in alkali borate binary glasses,” J. Mol. Struct. 993 (1–3), 155–159 (2011).
Funding
This work was performed in the framework of a state assignment of the Institute of Metallurgy, Ural Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Ivanov, A.V., Ryabov, V.V. Viscosity of the Sodium–Borate Melts Containing Mechanically Activated Samarium, Europium, Erbium, and Thulium Oxides. Russ. Metall. 2021, 133–136 (2021). https://doi.org/10.1134/S0036029521020105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029521020105