Abstract
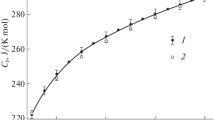
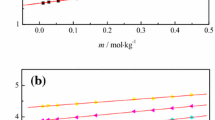
The isobaric heat capacities of phenol and its aqueous solutions with concentrations of 2, 4, and 5.9 wt % were studied using a scanning calorimeter (IT-s-400) at temperatures from 313 to 473 K and pressures of up to 19.6 MPa. The results were compared with the literature data in the given range of state parameters.




Similar content being viewed by others
REFERENCES
N. B. Vargaftic, Tables on the Thermophysical Properties of Liquids and Gases (Nauka, Moscow, 1972; Halsted Press, New York, 1975).
Thermophysical Properties of Fluid Systems. http://webbook.nist.gov/chemistry/fluid/
M. Frenkel, R. Chirico, V. Diky, et al., NIST Thermo Data Engine, NIST Standard Reference Database 103b-Pure Compound, Binary Mixtures, and Chemical Reactions, Vers. 5.0 (Natl. Inst. Stand. Technol., Boulder, CO, 2010).
G. S. Parks, H. Huffman, and M. Barmore, J. Am. Chem. Soc. 55, 2733 (1933).
A. N. Campbell and A. J. R. Campbell, J. Am. Chem. Soc. 62, 291 (1940).
R. J. L. Andon, J. F. Counsell, E. F. G. Herington, et al., Trans. Faraday Soc. 59, 830 (1963).
Yu. L. Rastorguev and Yu. A. Ganiev, Izv. Vyssh. Uchebn. Zaved., Neft Gaz 10, 79 (1967).
N. Nichols and I. Wads, J. Chem. Thermodyn. 7, 329 (1975).
G. Perron and J. E. Desnoyers, Fluid Phase Equilib. 2, 239 (1979).
M. L. Origlia-Luster, K. Ballerat-Busserolles, E. D. Merkley, et al., J. Chem. Thermodyn. 35, 331 (2003).
M. Censky, L. Hnedkovsky, and V. Majer, J. Chem. Thermodyn. 37, 205 (2005).
V. Hynek, L. Hnedkovsky, and I. Cibulka, J. Chem. Thermodyn. 29, 1237 (1997).
C. M. Criss and R. H. Wood, J. Chem. Thermodyn. 28, 723 (1996).
M. Censky, J. Sedlbauer, V. Majer, et al., Geochim. Cosmochim. Acta 71, 580 (2007).
R. A. Usmanov, R. R. Gabitov, S. A. Biktashev, et al., Russ. J. Phys. Chem. B 5, 1216 (2011).
Z. I. Zaripov, A. U. Aetov, R. R. Nakipov, et al., J. Mol. Liq. 307, 112935 (2020).
Z. I. Zaripov, A. U. Aetov, R. R. Nakipov, et al., J. Chem. Thermodyn. 152, 106270 (2021).
W. Wagner and A. Pruß, J. Phys. Chem. Ref. Data 31, 387 (2002).
E. W. Lemmon, M. L. Huber, and M. O. McLinden, NIST Standard Reference Database 23, NIST Reference Fluid Thermodynamic and Transport Properties, REFPROP, Vers. 10.0, Standard Reference Data Program (NIST, Gaithersburg, MD, 2018).
Y. M. Naziev, A. N. Shakhverdiev, M. M. Bashirov, et al., High Temp. 32, 936 (1994).
V. L. Gurvich and N. P. Sosnovskii, Selective Solvents in Petroleum Refining (Gos. Izdat. Neft. Gorno-Topl. Liter., Moscow, 1953) [in Russian].
M. Zabransky, Z. Kolska, V. Ruzicka, et al., J. Phys. Chem. Ref. Data 39, 404 (2010).
M. Zabransky and V. Ruzicka, J. Phys. Chem. Ref. Data 33, 1071 (2004).
Funding
This study was financially supported by the Russian Science Foundation (project no. 22-19-00117; https://rscf.ru/prjcard_int?22-19-00117).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaripov, Z.I., Nakipov, R.R., Mazanov, S.V. et al. Heat Capacity of Phenol and Its Aqueous Solutions at High Temperatures and Pressures. Russ. J. Phys. Chem. (2024). https://doi.org/10.1134/S0036024424020225
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036024424020225