Abstract
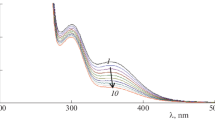
Kinetic investigation of Ru(III) catalyzed oxidation of menthol in an acidified solution of potassium bromate in the presence of Hg(OAc)2 as a scavenger of bromine intermediate has been studied. The reaction exhibits zero order kinetics with respect to KBrO3. While first order kinetics with respect to menthol and Ru(III) respectively. Hydrogen ion does not affect the rate but rate is enhanced by ionic strength. The influence of Hg(OAc)2 on the rate was found to be insignificant. Menthone as the oxidation product of menthol has been confirmed spectrally. The proposed mechanism involves slow and rate-controlling disproportionate of a transient complex formed between reactive species of Ru(III) and menthol. The various activation parameter such as energy of activation (∆E)#, Arrhenius factor (A), and entropy of activation (∆S)# were calculated. The rate law derived on the basis of experimental data corresponds well with the proposed mechanism.


Similar content being viewed by others
REFERENCES
J. C. Sullivan and R. C. Thompson, Inorg. Chem. 18, 2375 (1979).
P. Herbine and R. J. Field, J. Phys. Chem. 84, 1330 (1980).
M. Orbaw, F. Dc. Kepper, and I. R. Epstein, J. Am. Chem. Soc. 104, 1657 (1982).
S. B. Jonnalagadda, N. M. Munkombove, P. Hensman, and T. Mushinga, Int. J. Chem. Kinet. 23, 113 (1991).
S. B. Jonnalagadda, Anal. Chem. 55, 2253 (1983).
L. Farkas, B. Perlmutter, and O. Sehachter, J. Am. Chem. Soc. 71, 2833 (1949).
B. F. Mirjalil, M. A. Zolfigol, A. Bamoniri, et al., Acta Chim. Slov. 50, 563 (2003).
L. Farkas and O. Schachter, J. Am. Chem. Soc. 71, 2827 (1949).
S. Kajigaeshi, T. Nakagawa, N. Nagasaki, et al., Bull. Chem. Soc. Jpn. 59, 747 (1986).
A. Behr and K. Eustweweicmann, J. Organomet. Chem. 40, 3209 (1995).
H. Tomioka, K. Oshima, and H. Nozaki, Tetrahedron Lett. 25, 539 (1982).
S. Kanemoto, H. Tomioka, K. Oshima, and H. Nozaki, Bull. Chem. Soc. Jpn. 59, 105 (1986).
Y. Yamamoto, H. Sujuki, and Y. Mora-oka, Tetrahedron Lett. 26, 2107 (1985).
K. Takase, H. Masuda, O. Kai, et al., Chem. Lett., 871 (1995).
A. Banerjee, S. Dutt, D. Sengupta, et al., J. Ind. Chem. Soc. 60, 275 (1983).
L. Metsger and S. Binner, Tetrahedron Lett. 56, 1905 (2000).
T. L. Ho, Synth. Commun. 9, 237 (1979).
A. Banerjee, G. C. Banerjee, S. Bhattacharya, et al., J. Ind. Chem. Soc. 58, 605 (1981).
M. M. Adak, G. C. Banerjee, and A. Banerjee, J. Ind. Chem. Soc. 62, 224 (1985).
E. S. Amis, G. Nolen, and A. Indelli, J. Am. Chem. Soc. 82, 3233 (1960).
S. B. Jonnalagadda, N. M. Munkombwe, P. Hensman, and T. Mushinga, Int. J. Chem. Kinet. 23, 113 (2004).
G. Singh, R. Sailani, C. L. Khandelwal, and P. D. Sharma, Int. J. Curr. Chem. 2, 45 (2011).
R. Sailani, D. Pareek, N. K. Soni, et al., Curr. Phys. Chem. 4, 290 (2014).
P. Sharma, R. Sailani, A. Meena, and C. L. Khandelwal, J. Chem. Res. 44, 295 (2020).
G. Singh, P. Jain, R. Sailani, et al., J. Ind. Chem. Soc. 87, 817 (2010).
S. Hemkar, R. Sailani, C. L. Khandelwal, and P. D. Sharma, J. Ind. Chem. Soc. 89, 513 (2012).
S. Hemkar, P. Sharma, C. L. Khandelwal, and P. D. Sharma, J. Korean Chem. Soc. 56, 28 (2012).
R. Sailani, M. Sharma, D. Pareek, et al., React. Kinet. Mech. Catal. 105, 249 (2012).
R. Sailani, D. Pareek, A. Meena, et al., Int. J. Chem. Sci. 16, 1 (2018).
M. Latshaw, J. Am. Chem. Soc. 47, 793 (1925).
R. Natrajan and M. Venkatasubramanian, Tetrahedron Lett. 10, 5021 (1969);
Tetrahedron 30, 2785 (1974).
P. N. Char, S. Sondu, B. Sethuram, and T. N. Rao, Ind. J. Chem. A 27, 31 (1988).
N. Krishna Murthy, Ch. S. Reddy, and E. V. Sundaram, Ind. J. Chem. A 28, 288 (1989).
Ch. S. Reddy, Collect. Czeeh. Chem. Commun. 53, 3138 (1988).
S. B. Jonnalagadda, R. H. Simoyi, and G. K. Muthakia, J. Chem. Soc. Perkin Trans. 2, 1111 (1988).
J. C. Bailar, The Chemistry of Co-ordination Compounds (Reinhold, New York, 1966), p. 4; E. Koros and M. Varga, J. Phys. Chem. 88, 4116 (1984);
M. Varga, L. Gyorgyi, and E. Koros, J. Phys. Chem. 89, 1019 (1985).
N. Kumiya, S. Nuji, and S. Murahashi, Chem. Commun., 65 (2001).
A. E. Mucientes, R. E. Gabaldon, F. J. Poblete, and S. Villarreal, J. Phys. Org. Chem. 17, 236 (2004).
S. B. Jonnelagadda, M. Shezi, and B. Pare, Int. J. Chem. Kinet. 35, 294 (2003).
ACKNOWLEDGMENTS
Acknowledgment is extended to the co-authors and Head of the Department of Chemistry, University of Rajasthan Jaipur (Rajasthan India). Authors would like to dedicate this work to Prof. (Late) P. D. Sharma, Department of Chemistry, University of Rajasthan, Jaipur, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jain, P., Rolaniya, A. & Sailani, R. Ru(III) Catalyzed Oxidation of Menthol by Bromate in Presence of Mercuric Acetate in Aqueous Acidic Medium: A Kinetic and Mechanistic Pathway. Russ. J. Phys. Chem. 96, 2381–2386 (2022). https://doi.org/10.1134/S0036024422110255
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422110255