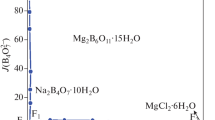Abstract
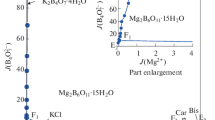
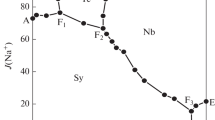
Solubility data for the quaternary system (KCl + MgCl2 + K2B4O7 + MgB4O7 + H2O) is very important for the separation of potassium and boron from the salt lake brines in Qaidam Basin. The solubilities and physicochemical properties including density and refractive index of the quaternary system (KCl + MgCl2 + K2B4O7 + MgB4O7 + H2O) at 318.15 K and 0.1 MPa were investigated experimentally with the method of isothermal dissolution equilibrium. In the phase diagrams of this quaternary system at 318.15 K, there are three invariant points, seven univariant isotherm dissolution curves, and five crystallization regions corresponding to KCl, MgCl2⋅6H2O, K2B4O7⋅4H2O, Mg2B6O11⋅15H2O and double salt KCl⋅MgCl2⋅6H2O, respectively. The solution density and refractive index of the quaternary system at 318.15 K change regularly with the increasing of Mg2+ concentration.




Similar content being viewed by others
REFERENCES
X. Y. Zheng, M. G. Zhang, Y. Xu, et al., Salt Lakes in China (Chin. Sci., Beijing, 2002).
S. Q. Wang, D. Zhao, Y. Song, X. M. Du, Y. F. Guo, and T. L. Deng, Russ. J. Phys. Chem. A 92, 2601 (2018).
L. Z. Meng, T. L. Deng, Y. F. Guo, and D. Li, Russ. J. Phys. Chem. A 86, 1526 (2012).
L. Z. Meng and D. Li, Braz. J. Chem. Eng. 31, 251 (2014).
Y. F. Guo, S. R. Sun, D. L. Gao, et al., Acta Geol. Sin.-Engl. 88 (S1), 326 (2014).
S. Q. Wang, X. M. Du, Y. Jing, et al., J. Chem. Eng. Data 62, 253 (2017).
D. C. Li, J. S. Yuan, and S. Q. Wang, Russ. J. Phys. Chem. A 88, 42 (2014).
T. L. Deng, S. Q. Wang, and B. Sun, J. Chem. Eng. Data 53, 411 (2008).
X. D. Yu, Y. Zeng, S. S. Guo, et al., J. Chem. Eng. Data 61, 1246 (2016).
C. Fu, S. H. Sang, M. F. Zhou, et al., J. Chem. Eng. Data 61, 1071 (2016).
S. Y. Gao, K. F. Xu, G. Li, et al., Acta Chim. Sin. 44, 1229 (1986).
H. Wang, L. Li, L. M. X. Wang, et al., J. Chem. Eng. Data 62, 3334 (2017).
S. Q. Wang, D. Zhao, Y. Song, X. M. Du, Y. F. Guo, and T. L. Deng, Russ. J. Phys. Chem. A 92, 2601 (2018).
S. Q. Wang, D. Zhao, Y. Song, et al., Russ. J. Inorg. Chem. 64, 661 (2019).
S. Q. Wang, X. M. Du, Y. Jing, Y. F. Guo, and T. L. Deng, Russ. J. Phys. Chem. A 91, 2503 (2017).
S. Q. Wang, Y. Song, X. M. Du, et al., Russ. J. Inorg. Chem. 63, 116 (2018).
S. Q. Wang, C. C. Shi, F. Yuan, et al., J. Chem. Eng. Data 65, 49 (2020).
D. C. Li, R. Fan, S. N. Yang, et al., Chem. Res. Chin. Univ. 34, 803 (2018).
Analytical Laboratory of Qinghai Inst. of Salt Lakes at CAS, The Analyses of Brines and Salts, 2nd ed. (Chin. Scientific, Beijing, 1988).
ACKNOWLEDGMENTS
The work was supported by the Program of the National Natural Science Foundation of China (22078247, U1707602, and U1507109), the Natural Science Foundation of Hebei Province (B2021202058), the Natural Science Foundation of Tianjin (17JCYBJC19500), and the Yangtze Scholars and Innovative Research Team in Chinese University (IRT_17R81) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, Jt., Zhang, L., Li, Dc. et al. Solubilities, Densities, and Refractive Indices of the Quaternary System (KCl + MgCl2 + K2B4O7 + MgB4O7 + H2O). Russ. J. Phys. Chem. 95 (Suppl 2), S217–S221 (2021). https://doi.org/10.1134/S0036024421150243
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421150243