Abstract
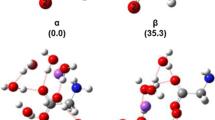
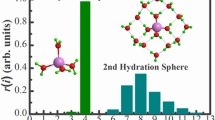
Micro hydration structures of the sodium ion, [Na(H2O) n ]+, n = 1–12, were probed by density functional theory (DFT) at B3LYP/aug-cc-pVDZ level in both gaseous and aqueous phase. The predicted equilibrium sodium–oxygen distance of 0.240 nm at the present level of theory. The four-, five- and six-coordinated cluster can transform from each other at the ambient condition. The analysis of the successive water binding energy and natural charge population (NBO) on Na+ clearly shows that the influence of Na+ on the surrounding water molecules goes beyond the first hydration shell with the hydration number of 6. The Car-Parrinello molecular dynamic simulation shows that only the first hydration sphere can be found, and the hydration number of Na+ is 5.2 and the hydration distance (rNa–O) is 0.235 nm. All our simulations mentioned in the present paper show an excellent agreement with the diffraction result from X-ray scattering study.
Similar content being viewed by others
References
P. R. Smirnov and V. N. Trostin, Russ. J. Gen. Chem 78, 1643 (2008).
X. Li, Y. Tu, H. Tian, and H. Agren, J. Chem. Phys. 132, 104505 (2010).
Y. Liu, H. Lu, Y. Wu, T. Hu, and Q. Li, J. Chem. Phys. 132, 124503 (2010).
Y. Wang, H. Yi, H. Li, Q. Dai, Z. Cao, and Y. Lu, Acta Phys. Chim. Sin. 31, 1035 (2015).
J. Lu, Y. Yu, and Y. Li, Fluid Phase Equilib. 85, 81 (1993).
Y. Yu, G. Gao, and Y. Li, Fluid Phase Equilib. 173, 23 (2000).
Y. Yu, G. Gao, J. Daridon, and B. Lagourette, Fluid Phase Equilib. 206, 205 (2003).
W. Xie and Y. Gao, J. Phys. Chem. Lett. 4, 4247 (2013).
H. Ohtaki and T. Radnai, Chem. Rev. 93, 1157 (1993).
P. R. Smirnov and V. N. Trostin, Russ. J. Gen. Chem. 77, 844 (2007).
S. Varma and S. B. Rempe, Biophys. Chem. 124, 192 (2006).
T. Megyes, S. Bálint, T. Grósz, T. Radnai, I. Bakó, and P. Sipos, J. Chem. Phys. 128, 044501 (2008).
T. Megyes, S. Bálint, E. Peter, T. Grósz, I. Bakó, and H. Krienke, J. Phys. Chem. B 113, 4054 (2009).
Y. Zhou, C. Fang, Y. Fang, F. Zhu, S. Tao, and S. Xu, Russ. J. Phys. Chem. A 86, 1236 (2012).
Y. Zhou, C. Fang, and Y. Fang, Acta Phys. Chim. Sin. 26, 2323 (2010).
J. Mähler and I. Persson, Inorg. Chem. 51, 425 (2012).
A. Bankura, V. Carnevale, and M. L. Klein, Mol. Phys. 112, 1448 (2014).
S. S. Azam, H. Zaheerul, and M. Q. Fatmi, J. Mol. Liq. 153, 95 (2010).
R. Mancinelli, A. Botti, F. Bruni, M. A. Ricci, and A. K. Soper, J. Phys. Chem. B 111, 13570 (2007).
A. Bankura, V. Carnevale, and M. L. Klein, J. Chem. Phys. 138, 014501 (2013).
A. C. Olleta, H. M. Lee, and K. S. Kim, J. Chem. Phys. 124, 024321 (2006).
A. C. Olleta, H. M. Lee, and K. S. Kim, J. Chem. Phys. 126, 144311 (2007).
J. S. Rao, T. C. Dinadayalane, J. Leszczynski, and G. N. Sastry, J. Phys. Chem. A 112, 12944 (2008).
T. H. Dunning, J. Chem. Phys. 90, 1007 (1989).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Revision A.01 (Gaussian Inc., Wallingford, CT, 2009).
CPMD V3.11 CIC (MPI, 1997–2001).
A. D. Becke, Phys. Rev. A 38, 3098 (1988).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
N. Troullier and J. L. Martins, Phys. Rev. B 43, 1993 (1991).
F. Xia, H. Yi, and D. Zeng, J. Phys. Chem. A 114, 8406 (2010).
Y. Zhou, Y. Fang, C. Fang, F. Zhu, H. Ge, and Q. Chen, J. Phys. Chem. B 117, 11709 (2013).
K. G. Spears and S. H. Kim, J. Phys. Chem. 80, 673 (1976).
C. Peng, P. Y. Ayala, H. B. Schlegel, and M. J. Frisch, J. Comput. Chem. 17, 49 (1996).
S. Reiser, S. Deublein, J. Vrabec, and H. Hasse, J. Chem. Phys. 140, 044504 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Yongquan, Z., Chunhui, F., Yan, F. et al. Reconsideration on Hydration of Sodium Ion: From Micro-Hydration to Bulk Hydration. Russ. J. Phys. Chem. 91, 2539–2547 (2017). https://doi.org/10.1134/S0036024417130313
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024417130313