Abstract
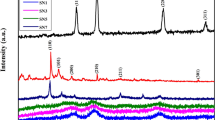
Calcium oxide (CaO) and tin oxide (SnO2) with nanodimension have attracted significant attention as effective chemosorbents for toxic compounds. Hydrothermal method was used for the synthesis of highly porous CaO-SnO2 nanocomposites by using sodium dodecylsulfate surfactant as a templating agent. Effect of concentration of surfactant as well as its critical micelle concentration (CMC) on the particle size of the nanocomposites was investigated. X-ray diffraction (XRD) was used to determine the crystal phases of these nanocomposites and particle size and morphology was identified by using transmission electron microscopy (TEM), scanning electron microscopy-energy dispersive X-ray (SEM/EDX) respectively. Furthermore thermo gravimetric analysis (TGA) and fourier transform infrared (FTIR) spectroscopy techniques were also used to characterize the CaO-SnO2 nanocomposites.
Similar content being viewed by others
References
G. Patel, U. Pal, and S. Menon, Sep. Sci. Technol. 44, 2806 (2009).
M. A. Farrukh, B. T. Heng, and R. Adnan, Turk. J. Chem. 34, 537 (2010).
D. Gouvea, G. J. Pereirab, L. Gengembrec, M. C. Steil, R. Pascal, A. Rubbensc, P. Hidalgoa, and R. H. R. Castroe, Appl. Surf. Sci. 257, 4219 (2011).
M. Shahid, M. A. Farrukh, A. A. Umar, and M. Khaleeq-ur-Rahman, Russ. J. Phys. Chem. A 88, 836 (2014).
M. Zabeti, W. M. A. Wan Daud and M. A. Kheireddine, Appl. Catal. A: Gen. 366, 154 (2009).
A. Demirbas, Energy Convers. Manage. 48, 937 (2007).
M. L. Granados, M. D. Z. Poves, D. M. Alonso, R. Mariscal, F. C. Galisteo, T. R. Moreno, J. Santamaria, and J. L. G. Fierro, Appl. Catal. B: Environ. 73, 317 (2007).
Z. X. Tang, Yu Zhen, Xin-Yi Zhang, Qin-Qin Pan, and Lu-E Shi, Quim. Nova. J. 2, 19 (2013).
N. A. Oladoja, I. Ololade, S. E. Olaseni, V. O. Olatujoye, O. S. Jegede, and A. O. Agunloye, Ind. Eng. Chem. 51, 639 (2012).
S. L. Martinez, R. Romero, J. C. Lopez, A. Romero, V. S. Mendieta, and R. Natividad, Ind. Eng. Chem. 50, 2665 (2011).
G. B. Cai, G. X. Zhao, X. K. Wang, and S. H. Yu, J. Phys. Chem. C 114, 12948 (2010).
M. M. Najafpour, S. Nayeri, and B. Pashaei, Dalton. Trans. 40, 9374 (2011).
Z. Wan and B. H. Hameed, Bioresour. Technol. 102, 2659 (2011).
S. Ferrere, A. Zaban and B. A. Gregg, J. Phys. Chem. B 101, 4490 (1997).
T. Hayakawa and M. Nogami, Sci. Technol. Adv. Mater. 6, 66 (2005).
M. A. Farrukh, P. Tan, and R. Adnan, Turk. J. Chem. 36, 303 (2012).
M. B. Sahana, C. Sudakar, A. Dixit, J. S. Thakur, R. Naik, and V. M. Naik, Acta Mater. 60, 1072 (2012).
R. Adnan, N. A. Razana, I. A. Rahman and M. A. Farrukh, J. Chin. Chem. Soc. 57, 222 (2010).
R. Gavagnin, L. Biasetto, F. Pinna, and G. Strukul, Appl. Catal. B: Environ. 38, 91 (2000).
H. Perveen, M. A. Farrukh, M. Khaleeq-ur-Rahman, B. Munir, and M. A. Tahir, Russ. J. Phys. Chem. A 89, 99 (2015).
T. E. Moustafid, H. Cachet, B. Tribollet, and D. Festy, Electrochim. Acta 47, 1209 (2007).
M. Okuya, S. Kaneko, K. Hiroshima, I. Yaggi, and K. Murakami, J. Eur. Ceram. Soc. 21, 2099 (2001).
F. L. Chen and M. L. Liu, Chem. Commun., 1829 (1999).
C. Kim, M. Noh, M. Choi, J. Cho, and B. Park, Chem. Mater. 17, 3297 (2005).
P. T. Wierzchowski and W. L. Zatorski, Appl. Catal. B: Environ. 44, 53 (2003).
L. Chou, Y. Cai, B. Zhang, J. Niu, S. Ji, and S. Li, Appl. Catal. A: Gen. 238, 185 (2003).
H. C. Wang, Y. Li, and M. J. Yang, Sens. Actuators B: Chem. 119, 380 (2006).
Z. J. Li, Z. Qin, Z. H. Zhou, L. Y. Zhang, and Y. F. Zhang, Nanoscale Res. Lett. 4, 1434 (2009).
M. R. Vaezi and S. K. Sadrnezhaad, Mater. Sci. Eng. B: Solid 140, 73 (2007).
S. Somita and R. Roy, Bull. Mater. Sci. 23, 453 (2000).
H. Zhu, Y. Wang, N. Wang, Y. Li, and J. Yang, J. Mater. Lett. 58, 2631 (2004).
Z. Mirghiasi, F. Bakhtiari, E. Darezereshki, and E. Esmaeilzadeh, J. Ind. Eng. Chem. 20, 113 (2014).
A. Roy and J. Bhattacharya, Int. J. Nanosci. 10, 413 (2011).
A. Imtiaz, M. A. Farrukh, M. K. Rahman, and R. Adnan, Sci. World J. 2013, Article ID 641420 (2013).
Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa, and T. Miyasaka, Science 276, 1395 (1997).
Q. Dong, H. Su, D. Zhang, N. Zhu, and X. Q. Guo, Acta Mater. 13, 3146 (2008).
Z. Pan, Z. Dai, and Z. L. Wang, Science 291, 1947 (2001).
Z. Q. Liu, D. H. Zhang, S. Han, C. Li, T. Tang, W. Jin, X. L. Liu, B. Lei, and C. W. Zhou, Adv. Mater. 15, 1754 (2001).
D. F. Zhang, L. D. Sun, J. L. Yin, and C. H. Yan, Adv. Mater. 15, 1022 (2003).
Z. R. Dai, Z. W. Pan, and Z. L. Wang, Solid State Commun. 118, 351 (2001).
A. Mansoori, T. R. Bastami, A. Ahmadpur, and Z. Eshaghi, Ann. Rev. Nano. Res. 2, 439 (2008).
V. Stengl, J. Subrt, P. Bezdicka, M. Marikova, and S. Bakadjieva, Solid State Phenom. 90, 121 (2003).
D. Vujicić, A. Comić, R. Zarubica, R. Micić, and G. Bosković, Fuel 89, 2054 (2010).
I. Muneer, M. A. Farrukh, S. Javaid, M. Shahid, and M. Khaleeq-ur-Rahman, Superlat. Microstruct. 77, 256 (2015).
E. A. Dean, Acta. Crystallogr. B 66, 271 (2010).
Y. C. Sharma, B. Singh, and J. Korstad, Energ. Fuel 24, 3223 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Munir, B., Farrukh, M.A., Perveen, H. et al. Template assisted synthesis of CaO-SnO2 nanocomposites. Russ. J. Phys. Chem. 89, 1051–1058 (2015). https://doi.org/10.1134/S0036024415060059
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024415060059