Abstract
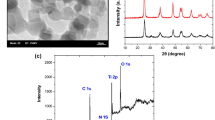
The development of industry induced a massive increase in the emission of carbon dioxide into the atmosphere. A large amount of CO2 and its general availability causes that it could be a cheap reactant in a reaction that runs in a way similar to photosynthesis in plants. Pure TiO2 and metal doped TiO2 are the most studied semiconductor catalysts for photoreduction of CO2. The TiO2/SiO2 and Pd/TiO2/SiO2 catalysts were prepared and studied by temperature-programmed desorption, X-ray diffraction analysis, SEM-EDS, temperature-programmed reduction and then used for the methanol synthesis. The photoactivity of Pd/TiO2/SiO2 catalysts in the reduction of CO2 with H2O was tested at room temperature using photoreactor equipped with 16 lamps. The wavelength was characteristic of near ultraviolet. Post-reaction products were identified with gas chromatograph equipped with the flame ionization detector. Pd doping made the catalysts photoactive and the photoactivity of catalysts was changing as follows: 1%Pd/5%TiO2/SiO2 > 1% Pd/10% TiO2/SiO2 > 1% Pd/15% TiO2/SiO2. Optimum ultraviolet radiation time in the photoreduction of CO2 to methanol was 7 h. An addition of Pd does not change the surface of the carrier.
Similar content being viewed by others
References
Carbon Dioxide Information Analysis Center: United Nations Statistics Division, 2006).
M. Halmann, Energy Resources Through Photochemistry and Catalysis, Ed. By M. Griitzel (Academic Press, New York, 1983), Ch. 15.
M. Halmann, Chemical Fixation of Carbon dioxide: Methods for Recycling CO2 into Useful Products (CRC Press, N.Y., 1993).
H. Slamet, H. W. Nasution, E. Purnama, et al., Catal. Commun. 6(5), 313 (2005).
M. Watanabe, Surf. Sci. 279(3), L236 (1992).
O. Ishitani, C. Inoue, Y. Suzuki, et al., J. Photochem. Photobiol., Ser. A 72(3), 269 (1993).
I.-H. Tseng, W.-C. Chang, and J. C. S. Wu, Appl. Catal., Ser. B 37(1), 37 (2002).
H. Yamashita, Y. Fujii, Y. Ichihashi, et al., Catal. Today 45(1–4), 221 (1998).
S. Kuwabata, K. Nishida, R. Tsuda, et al., J. Electrochem. Soc. 141(6), 1498 (1994).
K. Hirano, K. Inoue, and T. Yatsu, J. Photochem. Photobiol., Ser. A 64(2), 255 (1992).
E. Kocioek-Balawejder and M. Szymczyk, Przem. Chem. 86(12), 1179 (2007).
S. Kaneco, Y. Shimizu, K. Ohta, et al., J. Photochem. Photobiol., Ser. A 115(3), 223 (1998).
M. Anpo, Catal. Surv. Jpn. 1(2), 169 (2004).
A. Fujishima, T. N. Rao, and D. A. Tryk, J. Photochem. Photobiol., Ser. C 1(1), 1 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Zbudniewek, K., Góralski, J. & Rynkowski, J. Studies on TiO2/SiO2 and Pd/TiO2/SiO2 Catalysts in Photoreduction of CO2 with H2O to Methanol. Russ. J. Phys. Chem. 86, 2057–2062 (2012). https://doi.org/10.1134/S0036024412130274
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024412130274