Abstract
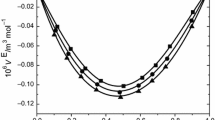
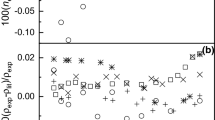
Densities, viscosities, refractive indices and ultrasonic velocities of the binary mixtures of acetophenone with ethyl acetate were measured over the entire mole fractions at 303.15, 313.15, and 323.15 K. From the experimental results, excess molar volumes V E, viscosity deviation Δη, refractive index deviation Δn D , deviations in isentropic compressibility Δκ s and excess intermolecular free length ΔL f are calculated. The viscosity values were fitted to the models of Krishnan-Laddha and McAllister. The thermophysical properties under study were fit to the Jouyban-Acree model. The excess values were correlated using Redlich-Kister polynomial equation to obtain their coefficients and standard deviations. The data obtained fitted with the values correlated by the corresponding models very well. The results are interpreted in terms of molecular interactions occurring in the solution.
Similar content being viewed by others
References
M. B. Ewing, B. J. Levian, and K. N. Marsh, J. Chem. Thermodyn. 2, 689 (1970).
A. Mchaweh, A. Alsaygh, and M. A. Mosh-Feghian, Fluid Phase Equilib. 224, 157 (2004).
C. M. Kenart and W. Kenart, Phys. Chem. Liq. 38, 155 (2000).
K. Saravanakumar, R. Baskaran, and T. R. Kubendran, J. Solution Chem. 40, 955 (2011).
K. Saravanakumar, R. Baskaranand, and T. R. Kubendran, Asian J. Chem. 23, 2643 (2011).
K. Saravanakumar, R. Baskaran, and T. R. Kubendran, Russ. J. Phys. Chem., Ser. A (in press).
R. A. McAllister, AIChE J. 6, 427 (1960).
M. R. V. Krishnan and G. S. Laddha, Ind. Chem. Eng. Trans. 57 (1963).
A. Jouyban, M. Khoubnasabjafari, Z. Vaezgharamaleki, et al., J. Chem. Pharm. Bull. 53, 519 (2005).
Otto Redlich and A. T. Kister, Ind. Eng. Chem. 40, 341 (1948).
D. D. Perrin and W. L. F. Armarego, Purification of Laboratory Chemicals, 3rd. (Pergamon Press, Oxford, 1988).
J. A. Riddick, W. B. Bunger, and T. K. Sakano, Organic Solvents—Physical Properties and Methods of Purification, 4th ed. (Wiley-Interscience, New York, 1986).
A. J. Treszczanowicz, O. Kiyohara, and G. C. Benson, J. Chem. Thermodyn. 13, 253 (1981).
A. Roux and J. Desnoyers, Ind. Acad. Proc., Chem. Soc. 98, 435 (1978).
R. J. Fort and W. R. Moore, Trans. Faraday. Soc. 62,1112 (1966).
Y. Maham, L. G. Hepler, A. E. Mather, et al., J. Chem. Soc. Faraday Trans. 93, 1747 (1997).
S. L. Oswal and N. B. Patel, J. Chem. Eng. Data 45, 225 (2000).
G. V. Rama Rao, A. Viswanatha Sarma, and C. Rambabu, Ind. J. Pure Appl. Phys. 42, 820 (2004).
V. Syamala, P. Venkateshwarlu, and K. Sivakumar, J. Chem. Eng. Data 51, 928 (2006).
H. Iloukhani and Z. Rostami, J. Chem. Eng. Data 52,921 (2007).
M. M. Palaiologou, J. Chem. Eng. Data 41, 1036 (1996).
M. I. Aralaguppi, C. V. Jadar, and T. M. Aminabhavi, J. Chem. Eng. Data 44, 441 (1999).
T. M. Aminabhavi and K. Banerjee, J. Chem. Eng. Data. 43, 514 (1998).
José M. Resa, José M. Goenaga, Ana I. Sánchez-Ruiz, and Miguel Iglesias, J. Chem. Eng. Data 51(3) 1294 (2006).
Murali Krishna Patwari, Ranjith Kumar Bachu, Sathyanarayana Boodida, and Satyanarayana Nallani, J. Chem. Eng. Data 54(3) 1069 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Saravanakumar, K., Baskaran, R. & Kubendran, T.R. The variation of viscosity, refractive indices, compressibility, intermolecular free length, and excess molar volume of the acetophenone—ethyl acetate solutions at 303.15–323.15 K. Russ. J. Phys. Chem. 86, 1947–1952 (2012). https://doi.org/10.1134/S0036024412130195
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024412130195