Abstract
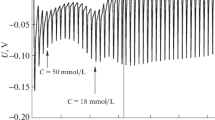
Conductivities, densities and ultrasonic speeds measurements of hexadecyltrimethylammonium bromide (HTAB) in aqueous solutions of glycine (Gly) and glycylglycine (Gly-Gly) have been made at various temperatures. The critical micelle concentration (CMC), the degree of ionization (β) of the micelles, standard free energy, enthalpy, and entropy of the micellization process (ΔG °m , ΔH °m , and ΔS °m ) for the present systems were estimated at different temperatures. The CMC values of HTAB in aqueous Gly and Gly-Gly were also evaluated by density and ultrasonic speed measurements. Apparent molar volumes, (V ϕ), apparent molar volumes at infinite dilution, (V °ϕ ), apparent molar compressibilities, (K ϕ), of HTAB in the pre- and post-micellar regions, and volume change on micellization (ΔV mϕ ) were also estimated. Large positive values of TΔS °m and small negative values of ΔH °m suggest that micellization process is driven primarily by entropy increase. The increase in ΔV mϕ and K ϕ with rise in temperature is indicative of less compact micellar structure of HTAB in presence of amino acid additives. These data suggest that amino acids are solubilised probably in the palisade layer of the micelle.
Similar content being viewed by others
References
Y. Moroi, Micelles, Theoritical and Applied Aspects (Plenum Press, New York, 1992).
A. Gonzalez-Perez, J. L. de Castillo, J. Czapkiewicz, and J. R. Rodriguez, J. Phys. Chem. B 105, 1720 (2001).
S. K. Mehta, K. K. Bhasin, R. Chauhan, and S. Dham, Colloids Surf. A: Physicochem. Eng. Aspects 255, 153 (2005).
R. De Lisi and S. Milioto, Chem. Soc. Rev. 4, 67 (1994).
E. Tovitou, F. L. Schaffer, N. Dayan, F. Alhaiquem, and F. Riccieri, Int. J. Pharm. 103, 131 (1994).
X. Qiu, W. Fang, Q. Lei, and R. Lin, J. Chem. Eng. Data 53, 942 (2008).
J. Jansen, C. Treiner, C. Vaution, and F. Pmisieux, Int. J. Pharm. 10, 319 (1994).
J. Chen, S. Shimura, K. Kirimura, and S. Usami, Biosci.: Biotechnol. Biochem. 58, 773 (1994).
S. K. Singh, A. Kunde, and N. Kishore, J. Chem. Thermodyn. 36, 7 (2004).
M. J. Rosen, Surfactants and Interfacial Phenomena (Wiley-Interscience, 1989).
R. G. Rayavarapu, C. Ungureanu, P. Krystek, T. G. van Leeuwen, and S. Manohar, Langmuir 26, 5050 (2010).
A. A. Vanin, E. M. Piotrovskaya, and N. A. Smirnova, Russ. J. Phys. Chem. A 81, 1256 (2007).
A. L. Lehninger, D. L. Nelson, and M. M. Cox, Principles of Biochemistry (Worth Publishers, USA, 1993).
A. Malliaris, J. Phys. Chem. 91, 6511 (1987).
C. R. Snelling, Density of Water (g/ml) vs. Temperature (°C), Handbook of Chemistry and Physics, 53rd ed. (2008).
A. K. Nain, A. Ali, and M. I. Alam, J. Chem. Thermodyn. 30, 1275 (1998).
A. Ali, S. Hyder, and A. K. Nain, J. Mol. Liq. 79, 89 (1999).
M. I. Aralaguppi, T. M. Aminabhavi, R. H. Balundgi, and S. S. Joshi, J. Phys. Chem. 95, 5299 (1991).
K. S. Sharma, S. R. Patil, A. K. Rakshit, K. Glenn, M. Doiron, R. M. Palepu, and P. A. Hassan, J. Phys. Chem. B 108, 12804 (2004).
P. Somasundaran, Encyclopedia of Surface and Colloid Science, 2nd ed. (Taylor & Francis, New York, 2006) Vol. 8, p. 6592.
D. F. Evans and H. Wennerstrom, The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet. (Wiley-VCH, USA, 1999).
G. B. Ray, S. Ghosh, and S. P. Moulik, J. Surfact. Deterg. 12, 131 (2009).
R. C. Bazito and A. E. Seond Omar, Langmuir 18, 4362 (2002).
A. K. Rakshit and B. Sharma, Colloid Polym. Sci. 28, 45 (2003).
L. Yu, T. Lu, Y. X. Luan, J. Liu, and G. Y. Xu, Colloids Surf. A 257, 375 (2005).
A. Ali and N. H. Ansari, J. Surfact. Deterg. 31, 441 (2010).
R. Zana, J. Colloid Interface Sci. 78, 330 (1980).
Z. N. Markina, L. P. Panicheva, and N. M. Zadymova, Colloid J. 59, 341 (1997).
P. Mukerjee, Adv. Coll. Interface Sci. 1, 242 (1967).
R. Zana, in Cationic Surfactants: Physical Chemistry, Ed. by D. H. Rubingh and P. M. Holland (Dekker, New York, 1991). Ch. 2.
R. Zielinski, S. Ikeda, H. Nomura, and S. Kato, J. Chem. Soc. Faraday Trans. 84, 151 (1988).
E. Kudryashov, T. Kapustina, S. Morrissey, V. Buckin, and K. Dawson, J. Colloid Interface Sci. 203, 59 (1998).
S. K. Mehta, S. Chaudhary, K. K. Bhasin, R. Kumar, and M. Aratono, ColloidsSurf A: Physicochem. Eng. Aspects 304, 88 (2007).
J. L. Del Castillo, J. Czapkiewicz, A. Gonzalez-Perez, and J. R. Rodriguez, Colloids Surf. A: Physicochem. Eng. Aspects 166, 161 (2000).
A. Gonzalez-Perez, J. M. Ruso, G. Prieto, and F. Sarmiento, Colloid Polym. Sci. 282, 1133 (2004).
K. S. Pitzer, Thermodynamics (Mc Graw-Hill. New York, 1995), p. 544.
F. J. Millero, Chem. Rev. 71, 147 (1971).
T. S. Brun, H. Hoiland, and E. Vikingstad, J. Colloid Interface Sci. 63, 89 (1978).
B. Tutaj, A. Gonzalez-Perez, J. Czapkiewicz, J. L. Del Castillo, and J. R. Rodriguez, J. Solution Chem. 30, 1101 (2002).
H. L. Friedman and C. V. Krishnan, Water -A Comprehensive Treatise, Ed. by F. Franks (Plenum Press, New York, 1973), Vol. 3, Chap. 1.
A. K. Mishra and J. C. Ahluwalia, J. Phys. Chem. 88, 86 (1984).
A. Gonzalez-Perez, J. L. Del Castillo, J. Czapkiewicz, and J. R. Rodriguez, J. Chem. Eng. Data 46, 709 (2001) [Colloid Polym. Sci. 282, 1133 (2004)].
K. Gracie, D. Turner, and R. Palepu, Can. J. Chem. 74, 1616 (1996).
J. Wang, Z. Yan, Y. Zhao, and F. Cui, J. Chem. Eng. Data 49, 1354 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Ali, A., Tasneem, S., Bidhuri, P. et al. Critical micelle concentration and self-aggregation of hexadecyltrimethylammonium bromide in aqueous glycine and glycylglycine solutions at different temperatures. Russ. J. Phys. Chem. 86, 1923–1929 (2012). https://doi.org/10.1134/S0036024412130031
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024412130031