Abstract
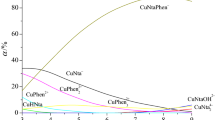
Thermal effects of the reactions of complex formation between d- and f-elements and 3,3′,5,5′-tetramethyl-4,4′-diethyl-2,2′-dipyrrolylmethene in dimethylformamide are determined from data of titration microcalorimetry and the temperature behavior of the complex formation equilibrium constants. The entropy and free energy changes of the complex formation reactions are calculated. It is shown that enthalpy-entropy compensation is observed for complex formation processes in all cases. Contributions from the enthalpy and entropy factors to the free energy change of the complex formation are analyzed, allowing us to confirm earlier conclusions as to the effect of the nature of a complexing agent on the relative percentage of covalent and ionic characters of the M-L bond. The dependence of the complex formation enthalpies on the inverse radius of a complexing agent is revealed for the lanthanide complexes.
Similar content being viewed by others
References
E. V. Antina and E. V. Rumyantsev, The Chemistry of Bilirubin and Its Analogues (KRASAND, Moscow, 2009) [in Russian].
E. V. Rumyantsev, A. Desoki, and E. V. Antina, Russ. J. Inorg. Chem. 54, 1735 (2009).
E. V. Rumyantsev, A. Desoki, and E. V. Antina, Russ. J. Inorg. Chem. 55, 932 (2010).
L. I. Martynenko, Russ. Chem. Rev. 60, 1008 (1991).
G. B. Guseva, E. V. Antina, M. B. Berezin, and A. I. V’yugin, Russ. J. Gen. Chem. 74, 1282 (2004).
A. S. Semeikin, S. A. Syrbu, M. B. Berezin, and T. V. Lyubimova, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 47(4), 10 (2004).
M. D. Taylor and C. P. Carter, J. Inorg. Nucl. Chem. 24, 387 (1960).
M. Beck and I. Nagepal, Chemistry of Complex Equilibria (Akademiai Kiado, Budapest, 1988).
V. P. Vasil’ev, Thermodynamic Properties of Solutions of Electrolytes (Vyssh. Shkola, Moscow, 1982) [in Russian].
K. B. Yatsimirskii, Theor. Exp. Chem. 16, 28 (1980).
K. B. Yatsimirskii, Theor. Exp. Chem. 12, 443 (1976).
G. B. Guseva, E. V. Antina, M. B. Berezin, et al., Russ. J. Phys. Chem. A 79, 908 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Rumyantsev, A. Desoki, E.V. Antina, T.R. Usacheva, V.A. Sharnin, 2012, published in Zhurnal Fizicheskoi Khimii, 2012, Vol. 86, No. 7, pp. 1168–1172.
Rights and permissions
About this article
Cite this article
Rumyantsev, E.V., Desoki, A., Antina, E.V. et al. Thermodynamic characteristics of complex formation reactions between d- and f-elements and alkylated dipyrrolylmethene in dimethylformamide. Russ. J. Phys. Chem. 86, 1053–1057 (2012). https://doi.org/10.1134/S0036024412070230
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024412070230