Abstract
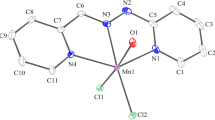
The protonation constants of the syn ketoxime, 2-(hydroxyimino)-1-(2-hydroxyphenyl)butane-1,3-dione (HPDO) were determined by a pH-metric method in solutions of dioxane, methanol and dimethylformamide (75 % v/v) at 20 ± 0.1 °C and ionic strength of 0.1 mol·L−1 supported by KNO3. The complexation reaction of the ligand HPDO with the transition metal ions Cu(II), Ni(II), Co(II), Cr(III), Fe(III), and Mn(II) in solution has been also studied potentiometrically. The stability constants of the respective species were determined and show that their values decrease in the order: 1,4-dioxane < DMF < methanol. The stability of the chelates is highly affected by the relative permittivity (ε r) and the donor number of the solvents. In addition, the complexes of [Cu(PDO)(Cl)]2·EtOH 1, [Ni(PDO)(H2O)(Cl)]2·2H2O 2, [Co(PDO)(Cl)]2·EtOH 3, [Fe(PDO)(Cl)2(EtOH)3]·EtOH 4, and [VO(PDO)H2O]2SO4·4H2O 5 were prepared and characterized by elemental analysis, IR, UV/Vis spectra, magnetic measurements and thermal analysis. Magnetic and spectral data suggest the formation of (i) neutral polymerized molecules accompanied by inclusion of lattice or coordinated water molecules, and (ii) a paramagnetic octahedral structure around metal ions in all cases. The geometry optimization of the syn structure of the ligand HPDO has been also calculated by HyperChem 8.0 software.










Similar content being viewed by others
References
Naskar, J.P., Biswas, C., Lu, L., Zhu, M.: Synthesis, crystal structure and spectroscopic properties of an oximato bridged Cu(II) dimer. J. Chem. Crystallogr. 41, 502–507 (2011)
Smith, A.G., Tasker, P.A., White, D.J.: The structures of phenolic oximes and their complexes. Coord. Chem. Rev. 241, 61–85 (2003)
Narasaka, K.: Synthesis of azaheterocycles from oxime derivatives. Pure Appl. Chem. 75, 19–28 (2003)
Pombeiro, A.J.L., Kukushkin, V.Y.: Reactions of coordinated nitriles. In: McClevery, J.A., Meyer, T.C. (eds.) Comprehensive Coordination Chemistry II, vol. 1, pp. 631–637. Elsevier, Amsterdam (2004)
Konidaris, K.F., Raptopoulou, C.P., Psycharis, V., Perlepes, S.P., Manessi-Zoupa, E., Stamatatos, T.C.: Use of the 2-pyridinealdoxime/N,N’-donor ligand combination in cobalt(III) chemistry: synthesis and characterization of two cationic mononuclear cobalt(III) complexes. Bioinorg. Chem. Appl. 2010, 1–7 (2010)
Cao, S., Zhang, M.: Color reaction of chromium(VI) with p-aminoN-N-diethylaniline in the presence of ethanol and cyclohexyldiamine tetraacetic acid as well as its application in the species analysis of chromium. J. Trace Microprobe Tech. 17, 157–164 (1999)
Soylak, M., Tuzen, M.: Diaion SP-850 resin as a new solid phase extractor for preconcentration–separation of trace metal ions in environmental samples. J. Hazard. Mater. B 137, 1496–1501 (2006)
Izquierdo, A., Compano, R., Granados, M.: Solvent extraction of 1-phenyl-1,2-propanedione-2-oxime and of its copper(II) and nickel(II) complexes into chloroform. Polyhedron 10, 919–926 (1991)
Izquierdo, A., Granados, M., Beltran, J.L.: Potentiometric study of complex formation equilibria of alpha-oxooximes with copper(II) and nickel(II) and ions. Talanta 39, 475–480 (1992)
Ando, I., Ujimto, K., Kurihara, H.: Protolytic kinetics of some pyridine derivatives in non-buffered aqueous solution. Bull. Chem. Soc. Jpn. 55, 713–716 (1982)
Shen, T., Xiao, R., Wang, Q., Yang, L., Wang, N.: Study of interactions and association thermodynamics between Sudan Red and sodium dodecyl sulfate in microemulsions. J. Dispers. Sci. Technol. 35, 435–440 (2014)
Rauf, M.A., Akhterb, Z., Kanwalb, S.: Photometric studies of the complexation of Sudan Red B with Mn+2 and Fe+3 ions. Dyes Pigm. 63, 213–215 (2004)
Dwyer, F.P., Mellor, D.P.: Chelating Agents and Metal Chelates. Academic Press Inc., London (1964)
Katritzky, A.R., Kuanar, M., Slavov, S., Hall, C.D.: Quantitative correlation of physical and chemical properties with chemical structure: utility for prediction. Chem. Rev. 110, 5714–5789 (2010)
Wittig, G.: Zur Erschließung der Benzo-γ-pyrone. Liebigs Ann. Chem. 446, 155–204 (1926)
Rossotti, F.J.C., Rossotti, H.: Potentiometric titrations using Grans plots. J. Chem. Ed. 42, 375–378 (1965)
Nakamoto, K.: Infrared Spectra of Inorganic and Coordination Compounds. Wiley, New York (1963)
König, E.: In: Hemmerich, P., Jørgensen, C.K., Neilands, J. B., Nyholm, R.S., Reinen, D., Williams, R.J.P. (eds.) Structure and Bonding, pp. 175–212. Springer, New York (1971)
Gray, H.B., Ballhausen, C.J.: A molecular orbital theory for square-planar metal complexes. J. Am. Chem. Soc. 85, 260–265 (1963)
Dilip, C.S., Thangaraj, V., Raj, A. P.: Synthesis, spectroscopic characterisation, biological and DNA cleavage properties of complexes of nicotinamide. Arabian J. Chem. (2011). doi:10.1016/j.arabjc/2011.07.016
Irving, H., Williams, R.J.P.: The stability of transition-metal complexes. J. Chem. Soc. 3192–3210 (1953)
Irving, H., Williams, R.J.P.: Order of stability of metal complexes. Nature 162, 746–747 (1948)
Irving, H., Rossotti, H. S.: Methods for computing successive stability constants from experimental formation curves. J. Chem. Soc. 3397–3405 (1953)
Mayer, U.: A semiemperical model for the description of solvent effects on chemical reactions. Pure Appl. Chem. 51, 1697–1712 (1979)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mosa, A.I., Ibrahim, M.M. & Abo-Melha, K.S. Spectroscopic and Thermodynamic Properties of Some Transition Metal Complexes Derived from 2-(Hydroxyimino)-1-(2-hydroxyphenyl)butane-1,3-dione. J Solution Chem 44, 2222–2235 (2015). https://doi.org/10.1007/s10953-015-0407-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0407-0