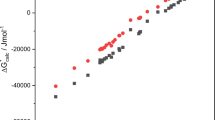
Abstract
A method for analyzing the thermodynamical manifestations of solvophobic effects is proposed on the basis of considering the relationship between the Gibbs energy and solvation enthalpy of nonelectrolytes. It is demonstrated that, for solutions in nonassociated solvents, there is a linear isoequilibrium dependence between them, and the coefficients of linear dependence are almost equivalent for various dissolved substances and solvents. It is determined that the deviations from this dependence observed in the case of associated solvents are always positive, and the consequences of the manifestations of solvophobic effects are considered. The contributions from the solvophobic effect to the Gibbs energy of solvation of various nonpolar compounds in methanol are determined on the basis of a thermodynamic model of solvation suggested earlier. It is shown that in both methanol and aqueous solutions, the values of these contributions correlate linearly with the characteristic molecular volume of the dissolved substance.
Similar content being viewed by others
References
C. Reichardt, Solvents and Solvent Effects in Organic Chemistry (Wiley, New York, 2004).
P. Ball, Chem. Rev. 108, 74 (2008).
W. Blokzijl and J. B. F. N. Engberts, Angew. Chem. 32, 1545 (1993).
M. N. Rodnikova and N. A. Chumaevskii, Zh. Strukt. Khim. 47, 154 (2006).
R. Palepu, H. Gharibi, D. M. Bloor, and E. Wyn-Jones, Langmuir 9, 110 (1993).
S. M. Leshchev, Zh. Fiz. Khim. 73, 58 (1999) [Russ. J. Phys. Chem. A 73, 52 (1999)].
M. H. Abraham, P. L. Grellier, and R. A. Mcgill, J. Chem. Soc., Perkin Trans. 2, No. 3, 339 (1988).
M. H. Abraham, M. J. Kamlet, and R. W. Taft, J. Chem. Soc., Perkin Trans., No. 8, 923 (1982).
M. H. Abraham, J. Am. Chem. Soc. 104, 2085 (1982).
R. Silveston and B. Kronberg, J. Phys. Chem. 93, 6241 (1989).
B. N. Solomonov and I. A. Sedov, Zh. Fiz. Khim. 82, 1259 (2008) [Russ. J. Phys. Chem. A 82, 1110 (2008)].
M. Sjoeberg, R. Silveston, and B. Kronberg, Langmuir 9, 973 (1993).
L. Liu and Q. X. Guo, Chem. Rev. 101, 673 (2001).
I. M. Barclay and J. A. V. Butler, Trans. Faraday Soc. 34, 1445 (1938).
A. R. Katritzky, A. A. Oliferenko, P. V. Oliferenko, et al., J. Chem. Inf. Comput. Sci. 43, 1806 (2003).
E. R. Thomas, B. A. Newman, T. C. Long, et al., J. Chem. Eng. Data 27, 399 (1982).
B. N. Solomonov, V. B. Novikov, M. A. Varfolomeev, and N. M. Mileshko, J. Phys. Org. Chem. 18, 49 (2005).
J. Li, T. Zhu, G. D. Hawkins, et al., Theor. Chem. Acc. 103(1), 9 (1999).
C. Mintz, K. Burton, J. Acree, and M. H. Abraham, QSAR Combinator. Sci. 27, 179 (2008).
B. N. Solomonov and I. A. Sedov, J. Phys. Chem. B 110, 9298 (2006).
B. N. Solomonov and I. A. Sedov, J. Mol. Liq. 139(1–3), 89 (2008).
I. A. Sedov and B. N. Solomonov, Zh. Fiz. Khim. 82, 817 (2008) [Russ. J. Phys. Chem. A 82, 704 (2008)].
M. D. Borisover, F. D. Baitalov, and B. N. Solomonov, J. Solution Chem. 24, 579 (1995).
D. M. Trampe and C. A. Eckert, J. Chem. Eng. Data 36, 112 (1991).
M. H. Abraham, G. S. Whiting, P. W. Carr, and H. Ouyang, J. Chem. Soc., Perkin Trans. 2, No. 6, 1385 (1998).
M. H. Abraham and J. C. McGowan, Chromatografia 23, 243 (1987).
M. H. Abraham, J. Andonian-Haftvan, G. S. Whiting, et al., J. Chem. Soc., Perkin Trans. 2, No. 8, 1777 (1994).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.A. Sedov, M.A. Stolov, B.N. Solomonov, 2011, published in Zhurnal Fizicheskoi Khimii, 2011, Vol. 85, No. 4, pp. 698–703.
Rights and permissions
About this article
Cite this article
Sedov, I.A., Stolov, M.A. & Solomonov, B.N. Evaluating the contribution of solvophobic effects to the Gibbs energy of solvation in methanol. Russ. J. Phys. Chem. 85, 621–626 (2011). https://doi.org/10.1134/S0036024411040236
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024411040236