Abstract
A method was tested to perform the solid-phase synthesis of ceramic wollastonite under hybrid microwave heating using such natural renewable raw materials as sea shells as a source of CaCO3 with the addition of commercial SiO2 powder. The XRD, SEM, TGA, and EDS methods were used to explore the effect of the mechanical homogenization time and the conditions for the chemical interaction of raw materials, provided that the required phase composition is reached. It was studied how temperature (800–1150°C) and time (15–60 min) of sintering affect the composition and structure of the ceramic wollastonite samples, including those in the presence of the strengthening additive sodium tetraborate. The formation of an apatite (Ca10(PO4)6(OH)2) layer on the surface of the obtained samples under conditions of their contact with artificial human blood plasma was assessed to confirm the biocompatible properties of these materials. The proposed method of synthesis is promising for obtaining a chemically pure valuable biomaterial in the form of synthetic wollastonite with the possibility of rational use of biogenic raw materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Inorganic materials occupy a key place in the creation of modern biomaterials, which are the basis of biotechnologies for the needs of personalized medicine [1]. The rapidly growing modern industry of biomaterials is designed to improve the quality of life and longevity of people. An important problem of chemical synthesis is to obtain high-purity biomaterials using available resource-saving technologies, in particular, those based on the use of natural renewable raw materials, including the resources of the oceans [2]. For example, sea sponges, corals, skeletons of sea urchins and cuttlefish, sea shells, and much more are excellent raw materials for inorganic compounds both for obtaining high-purity biocompatible materials [2, 3], and as templates for creating biostructures of complex architecture [4]. Similarly useful are plant raw materials or their processing waste, which contain valuable components for chemical synthesis: citrus fruits, cereals, nut, and other crops [5–7].
In this context, in this work, we considered the possibility of using such natural renewable raw materials as sea shells as a source of calcium carbonate (CaCO3) to synthesize various polymorphs of chemically pure wollastonite (CaSiO3). Synthetic wollastonite is widely used as a bioactive base and filler in the manufacture of ceramic implants for bone reconstructive and regenerative surgery in dentistry, orthopedics, maxillofacial surgery, etc. [8–10]. The demand for wollastonite for biomedical applications is determined by its hydrophilic nature, which favors the formation of an apatite layer on its surface under conditions of the bioorganic environment, into which Ca2+ and \({\text{SiO}}_{3}^{{2 - }}\) ions are intensely transferred [11–14]. It was proven that these ions can control regulate the proliferation, differentiation of osteoblasts, and expression of bone marker genes such as BMP-2, RUNX2, transforming growth factor (TGF-β), ALP, and osteocalcin [16]. Silicon ions are involved in the formation of the extracellular matrix and play a crucial role in the activation or deactivation of mitogen-activated protein kinase (MAPK) and the p38 MAPK signaling pathway [17]. These factors determine the bioactive properties of wollastonite, which activates osteoinduction and osteogenesis, and also binds to bone tissue through the process of “bonding osteogenesis,” as shown by Biswas et al. [18] and Maxim et al. [19], and also proved in our recent studies on in vitro and in vivo models [20–23].
Synthetic wollastonite with different morphology and microstructure can be obtained by the sol–gel, hydrothermal and, precipitation technologies [24–26]. These approaches are easy to perform and allow one to vary the size and shape of crystallites and the curvature and roughness of the surface. Such bulk structured materials as bioceramics based on wollastonite are successfully produced by consolidating powders by conventional methods of cold pressing with subsequent heat treatment [27], and hot and hot isostatic pressing [28], and also by an unconventional method of spark plasma sintering [29–32]. In this case, an attractive method is the solid-phase synthesis of wollastonite from a reaction mixture of raw components, e.g., by induction heating of a mixture of CaCO3 and SiO2 at 1320°C [33], or a mixture of CaO and SiO2 in various ratios at 1000–1200°C [34]. Natural components and/or various waste are also used: rice husk ash and eggshells at 850°C in the presence of a polyvinyl alcohol binder to produce ceramics [11], recycled glass and medical waste incinerator fly ash at 950°C [35], and boric acid production waste at 900–1000°C [36]. It was demonstrated to be obviously promising to use microwave heating to synthesize wollastonite from eggshells in the presence of a commercial SiO2 powder [37]. It was shown [37] that the initial temperature of the reaction of formation of wollastonite was 800°C, which makes this process superior in performance to the existing methods. In particular, of greater interest may be hybrid microwave heating accompanied by additional induction heating produced by using a microwave-energy-absorbing material placed in a microwave oven as a heating chamber, into which the raw material is put.
The purpose of this work was to study the solid-phase synthesis of ceramic wollastonite from such natural renewable raw materials as sea shells using hybrid microwave heating. The proposed method is promising due to the possibility of putting into use valuable bio raw materials to obtain chemically pure biomaterial by an environmentally attractive method of inorganic synthesis for solving important biomedical challenges.
EXPERIMENTAL
Reagents. Sea shells (Japanese scallop Chlamys farreri nipponensis), silicon oxide (SiO2), sodium tetraborate (Na2B4O7·7H2O), KCl, K2HPO4·3H2O, MgCl2, CaCl2, and Na2SO4, and tris(hydroxymethyl)aminomethane (TRIS).
Synthesis procedure. In the synthesis of wollastonite, a powder obtained from sea shells consisting mostly of calcium carbonate (CaCO3) was mixed with commercial SiO2 in a molar ratio of 1 : 1 to form a mixture homogenized by grinding in a planetary mill at 750 rpm. For the study, samples were taken at different grinding times of 15, 30, 60, 120, 180, 240, and 600 min. Next, 1-g powder samples obtained at 180, 240, and 600 min were subjected to uniaxial cold pressing at 200 MPa with subsequent microwave sintering in a Milestone flexiWAVE flexible microwave synthesis system. The system included two magnetrons with a generation frequency of 2.45 Hz and a maximum power of 2.4 kV, and a sintering chamber based on a microwave-absorbing silicon carbide material with sintering temperatures of 800–1200°C, a sintering rate of 20 deg/min, and a treatment time of 30–60 min. Additionally, a set of similar samples was obtained with the addition of 10 wt % sodium tetraborate to reduce the synthesis temperature.
Chemical reaction equation:
Method to assess the biocompatibility of samples. An assessment was made of the formation of an apatite (Ca10(PO4)6(OH)2) layer on the surface of ceramic wollastonite samples under conditions of their contact with artificial human blood plasma (AHBP). An AHBP solution was prepared according to the following procedure. Distilled water (700 mL) was poured into a plastic container with a flat bottom, and the reagents were dissolved in it in the following order: NaCl (8 g/L), NaHCO3 (0.35 g/L), KCl (0.224 g/L), K2HPO4·3H2O (0.228 g/L), MgCl2 (0.256 g/L), CaCl2 (0.278 g/L), Na2SO4 (0.071 g/L), and (CH2OH)3CNH2 (6.057 g/L). The last (eighth) reagent, TRIS, was added gradually to avoid turbidity of the solution. The pH was adjusted with a 1 M HCl solution within 7.4. Next, the AHBP solution was brought to the mark in a 1000-mL flask and used in the study.
The temperature of the solution was kept constant in a thermostat at 36°C. Ceramic samples were placed in portions of this solution and kept for 3 and 7 days;
Characteristics of methods of investigation. Phases in the obtained samples were identified by X-ray powder diffraction (XRD) with a D8 Advance Bruker AXS multipurpose X-ray diffractometer (Germany; CuKα radiation, Ni filter, average wavelength λ = 1.5418 Å, angle range 5°–80°, scanning step 0.02°, scanning speed 5 deg/min). Thermogravimetric analysis (TGA) was performed with a Shimadzu DTG-60H simultaneous DTA/TG apparatus (Japan) in platinum crucibles with pierced lids in a dry argon flow (20 mL/min) in the temperature range 35–1300°С at a heating rate of 10 deg/min. The structure of the studied materials was examined by scanning electron microscopy (SEM) on a CrossBeam 1540 XB Carl Zeiss electron microscope (Germany) with an energy-dispersive X‑ray spectroscopy (EDS) attachment.
RESULTS AND DISCUSSION
Effect of Mechanical Grinding Time
The study of the effect of the mechanical grinding time on the composition of the powder mixture based on CaCO3 and SiO2 showed that, early in the grinding, the sample is a mixture of crystalline phases Ca(OH2), CaO, and dominant phase CaCO3 (Fig. 1). With an increase in the grinding time, the intensity of the signals of the secondary phases decreases, whereas the main carbonate phase increasingly dominates and becomes the only phase in the sample after 10 h of treatment in the planetary mill. The long-term causes amorphization of the initial powders. In substances that are highly prone to be in a high-entropy state, nanostructuring under the action of mechanical grinding is often accompanied by amorphization because of the formation of structural defects and mechanical deformations during multiple interparticle collisions, tension, and crushing [38–40].
According to the XRD data, long-term mixing of the mixture is impractical, since it causes amorphization of the substance. In this case, the CaCO3 phase required for the synthesis is dominant in the mixture, and the Ca(OH)2 and CaO impurities also react with SiO2 during the formation of wollastonite.
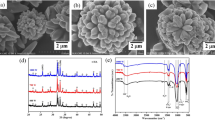
The SEM images show that the grinding of the samples in the planetary mill for 15 min leads to the formation of agglomerates that are no larger than 20 µm in size (Fig. 2a) and consist of nanometer-sized particles (Figs. 2b, 2c).
Effect of Time and Temperature of Heating
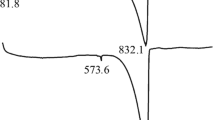
The conditions for the chemical interaction between the components of the initial mixture of CaCO3 and SiO2 according to equation (1) were studied by thermogravimetric analysis. The TGA curves show (Fig. 3) that the weight loss in the range from room temperature to 1200°C is 26.2%. These changes are accompanied by endothermic peaks in various temperature ranges in the sequence 135, 448, and 751°C and one exothermic event at 897°C. The first recorded weight loss (5.1%) up to 300°C is due to the removal of free and physically adsorbed water, as indicated by the endothermic peak centered around 135°C in the DTA curve. The second event of weight loss (6.4%) from 415°C to ~570°C can be attributed to the dehydroxylation of Ca(OH)2. This loss is accompanied by the endothermic peak at about 448°C in the DTA curve. Further weight loss (12.5%) in the temperature range from 600 to 800°C is accompanied by two endothermic peaks at 700 and 751°C. The first of them is due to the removal of CO2 during the decomposition of amorphous CaCO3, and the second characterizes the loss of CO2 during the decomposition of more crystalline CaCO3. The DTA curve also shows the exothermic peak at 897°C, which corresponds to the formation of crystalline wollastonite [32].
A preliminarily compressed initial mixture of CaCO3 and SiO2 that was obtained by grinding in the planetary mill for 15 min was sintered at 700, 800, 1000, and 1150°C under hybrid microwave heating conditions. The X-ray powder diffraction pattern (Fig. 4) shows that, with an increase in the microwave heating temperature, the intensity of the peak corresponding to the calcium oxide phase increases. This indicates the decomposition of CaCO3, which is in excess in the composition of the mixture. The formation of the α‑polymorph of wollastonite (α-CaSiO3) is observed at 1150°C; this sample has a higher mechanical strength and retains its integrity, unlike the other samples, which are mechanically unstable.
With an increase in the sintering time (15, 30, 60 min) of the mixture of CaCO3 and SiO2 at a maximum temperature of 1150°C, a mixture of α-CaSiO3 and β‑CaSiO3 wollastonite phases forms, and so does a calcium silicate Ca2SiO4 phase, as follows from the XRD data (Fig. 5).
The SEM study demonstrated that the ceramics obtained by sintering for 30 min has a porous structure formed by nanosized sintered grains (Figs. 6a, 6b). Large agglomerates and monolithic areas are not observed. Additional introduction of 10 wt % sodium tetraborate (borax) to the initial mixture before sintering leads to the formation of a nonporous sample with a monolithic structure (Figs. 6c, 6d). However, this additive increases the mechanical strength of the ceramics.
Figure 7 present the X-ray powder diffraction patterns of the ceramic samples, which show that the addition of sodium tetraborate favors the formation of the β- and α-polymorphs of wollastonite, but in the absence of an intermediate phase of calcium silicate Ca2SiO4.
Assessment of Biocompatibility of Ceramic Samples
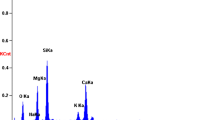
The assessment was made of the formation of an apatite (Ca10(PO4)6(OH)2) layer on the surface of the ceramic wollastonite samples under conditions of their contact with artificial human blood plasma. This assessment is an indirect proof of the biocompatibility and bioactivity of materials based on wollastonite, which are involved in the synthesis of apatite in a mineralized model blood solution. According to the obtained microscopy data (Fig. 8), the surface morphology of the ceramic wollastonite samples significantly changes after the treatment in the AHBP solution. After the treatment for 3 and 7 days, the new layer gradually grows on the surface of the samples and repeats the morphology of the original surface before the placement in the IPC solution. In the case of wollastonite with the porous structure formed by nanosized sintered grains (Figs. 6a, 6b), its surface is overgrown with a grained layer, which repeats the rounded shape of the particles (Figs. 8a, 8b). The surface of wollastonite with the addition of sodium tetraborate (Figs. 5c, 5d), on the contrary, is overgrown with a newly formed monolithic layer (Fig. 8b). According to the EDS data (Fig. 8), the newly formed, uniformly distributed, layer on the surface of the ceramic samples contains phosphorus and calcium, which corresponds to the formation of apatite synthesized upon contact with AHBP.
SEM images of the samples of the ceramics obtained from the mixture of CaCO3 and SiO2 (a, b) without additives and (c, d) in the presence of 10 wt % sodium tetraborate additive and treated in artificial human blood plasma for different times, and the EMF maps of the distribution of elements on the surface of these samples.
CONCLUSIONS
The method was tested to perform the solid-phase synthesis of ceramic wollastonite under hybrid microwave heating using such natural renewable raw materials as sea shells as a source of CaCO3 with the addition of commercial SiO2 powder. The XRD, SEM, TGA, and EDS studies showed the following. First, it was found that the optimal time of homogenization of the components of the raw material mixture (CaCO3 and SiO2) by mechanical grinding is 15 min, since an increase in this time to 10 causes gradual amorphization of CaCO3, inter alia, because of the nanosize of its particles. Second, the conditions for the chemical interaction of the components of the raw material mixture were determined. It was detected that the formation of crystalline wollastonite occurs at 897°C in air through the stage of decomposition of CaCO3 from sea shells to CaO and its reaction with SiO2. At the same time, the high heating temperature (1150°C) and the long treatment time (≥30 min) favor to form the mechanically strong ceramic, which retains its integrity, unlike the other obtained samples. Third, it was found that the addition of 10 wt % sodium tetraborate increases the mechanical strength of the ceramics; however, the structure of the obtained samples is monolithic, in contrast to the nanostructured ceramic framework of the samples produced without this strengthening component. Fourth, it was determined that the contact of the ceramic wollastonite samples with artificial blood plasma for 3 and 7 days changes their surface morphology because of the formation of an apatite layer. This indirectly confirms the biocompatibility and bioactivity of these materials, and also suggests that their further investigation is promising for solving problems of personalized medicine.
REFERENCES
M. Jemison and R. Olabisi, Acta Biomater. 128, 77 (2021). https://doi.org/10.1016/j.actbio.2021.04.033
M. Wan, W. Qin, C. Lei, et al., Bioact. Mater. 6, 4255 (2021). https://doi.org/10.1016/j.bioactmat.2021.04.028
A. Sibiya, J. Jeyavani, J. Sivakamavalli, et al., Reg. Stud. Mar. Sci. 44, 101760 (2021). https://doi.org/10.1016/j.rsma.2021.101760
N. P. Shapkin, E. K. Papynov, A. E. Panasenko, et al., Appl. Sci. 11 (2021). https://doi.org/10.3390/app11198897
N. Mahato, K. Sharma, M. Sinha, et al., J. Adv. Res. 23, 61 (2020). https://doi.org/10.1016/j.jare.2020.01.007
B. A. Goodman, J. Bioresour. Bioprod. 5, 143 (2020). https://doi.org/10.1016/j.jobab.2020.07.001
P. Sharma, V. K. Gaur, R. Sirohi, et al., Ind. Crops Prod. 152, 112550 (2020). https://doi.org/10.1016/j.indcrop.2020.112550
V. H. Ingole, B. Sathe, and A. V. Ghule, Fundam. Biomater. Ceram. 273 (2018). https://doi.org/10.1016/B978-0-08-102203-0.00012-3
R. Sainitya, M. Sriram, V. Kalyanaraman, et al., Int. J. Biol. Macromol. 80, 481 (2015). https://doi.org/10.1016/J.IJBIOMAC.2015.07.016
G. Bheemaneni, S. Saravana, and R. Kandaswamy, Mater. Today Proc. 5, 1807 (2018). https://doi.org/10.1016/J.MATPR.2017.11.279
S. Palakurthy, K. V.G.R., R. K. Samudrala, et al., Mater. Sci. Eng. C 98, 109 (2019). https://doi.org/10.1016/j.msec.2018.12.101
P. N. de Aza, F. Guitian, and S. de Aza, Scr. Metall. Mater. 31, 1001 (1994). https://doi.org/10.1016/0956-716X(94)90517-7
S. Ni and J. Chang, J. Biomater. Appl. 24, 139 (2009). https://doi.org/10.1177/0885328208094745
W. T. Barbosa, K. V. de Almeida, G. G. de Lima, et al., J. Biomed. Mater. Res. B 34462 (2019). https://doi.org/10.1002/jbm.b.34462
M. Y. Shie, S. J. Ding, and H. C. Chang, Acta Biomater. 7, 2604 (2011). https://doi.org/10.1016/J.ACTBIO.2011.02.023
L. Fei, C. Wang, Y. Xue, et al., J. Biomed. Mater. Res. B 100, 1237 (2012). https://doi.org/10.1002/JBM.B.32688
N. Biswas, A. Samanta, S. Podder, et al., J. Mech. Behav. Biomed. Mater. 86, 264 (2018). https://doi.org/10.1016/J.JMBBM.2018.06.046
L. D. Maxim, R. Niebo, M. J. Utell, et al., Inhal. Toxicol. 26, 95 (2014). https://doi.org/10.3109/08958378.2013.857372
E. K. Papynov, O. O. Shichalin, V. I. Apanasevich, et al., Prog. Nat. Sci. Mater. Int. 29, 569 (2019). https://doi.org/10.1016/J.PNSC.2019.07.004
E. K. Papynov, O. O. Shichalin, V. I. Apanasevich, et al., Powder Technol. 367, 762 (2020). https://doi.org/10.1016/J.POWTEC.2020.04.040
E. K. Papynov, O. O. Shichalin, V. I. Apanasevich, et al., Ceram. Int. 47, 22487 (2021). https://doi.org/10.1016/j.ceramint.2021.04.258
V. Apanasevich, E. Papynov, N. Plekhova, et al., J. Funct. Biomater. 11 (2020). https://doi.org/10.3390/JFB11040068
M. Guglielmi, G. Kickelbick, and A. Martucci, Sol-Gel Nanocomposites (Springer New York, New York, 2014).
P. Ros-Tarraga, A. Murciano, P. Mazon, et al., Ceram. Int. 43, 11034 (2017). https://doi.org/10.1016/J.CERAMINT.2017.05.146
K. Lin, J. Chang, G. Chen, et al., J. Cryst. Growth 300, 267 (2007). https://doi.org/10.1016/J.JCRYSGRO.2006.11.215
C. Vakifahmetoglu and L. Karacasulu, Curr. Opin. Solid State Mater. Sci. 24 (2020). https://doi.org/10.1016/j.cossms.2020.100807
Z. Matamoros-Veloza, K. Yanagisawa, J. C. Rendon-Angeles, et al., J. Phys. Condens. Matter 16 (2004). https://doi.org/10.1088/0953-8984/16/14/049
E. K. Papynov, V. Y. Mayorov, A. S. Portnyagin, et al., Ceram. Int. 41, 1171 (2015). https://doi.org/10.1016/J.CERAMINT.2014.09.045
E. K. Papynov, O. O. Shichalin, E. B. Modin, et al., RSC Adv. 6, 34066 (2016). https://doi.org/10.1039/c6ra04956g
E. K. Papynov, O. O. Shichalin, V. Y. Mayorov, et al., Ceram. Int. 43, 8509 (2017). https://doi.org/10.1016/J.CERAMINT.2017.03.207
E. K. Papynov, O. O. Shichalin, I. Y. Buravlev, et al., Russ. J. Inorg. Chem. 65, 263 (2020). https://doi.org/10.1134/S0036023620020138
Y. Hu, Z. Xiao, H. Wang, et al., Ceram. Int. 45, 3710 (2018). https://doi.org/10.1016/j.ceramint.2018.11.034
T. V. Vakalova, V. M. Pogrebenkov, and N. P. Karionova, Ceram. Int. 42, 16453 (2016). https://doi.org/10.1016/j.ceramint.2016.06.060
S. Palakurthy, K. V.G.R., R. K. Samudrala, et al., Mater. Sci. Eng. C 98, 109 (2019). https://doi.org/10.1016/0956-716X(94)90517-7
S. Papamarkou, C. Sifaki, P. E. Tsakiridis, et al., J. Environ. Chem. Eng. 6, 5812 (2018). https://doi.org/10.1016/j.jece.2018.09.006
S. B. Yarusova and I. Yu. B. Gordienko, et al., KnE Mater. Sci. 2020, 8135 (2020). https://doi.org/10.18502/kms.v6i1.8135
S. Vichaphund, M. Kitiwan, D. Atong, et al., J. Eur. Ceram. Soc. 31, 2435 (2011). https://doi.org/10.1016/j.jeurceramsoc.2011.02.026
R. B. Schwarz, R. R. Petrich, and C. K. Sau, J. Non-Cryst. Solids 76, 281 (1985).
Y. H. Zhao, Z. H. Jin, and K. Lu, Philos. Mag. Lett. 79, 747 (1999). https://doi.org/10.1080/095008399176814
L. Piot, S. Le Floch, T. Cornier, et al., J. Phys. Chem. C 117, 11133 (2013). https://doi.org/10.1021/jp401121c
ACKNOWLEDGMENTS
This work was performed using equipment of the United Center for Shared Facilities and Interdisciplinary Center for Nanotechnologies and New Functional Materials, Far Eastern Federal University, Vladivostok, Russia.
Funding
This work was supported by the Russian Science Foundation (project no. 18-73-10107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shichalin, O.O., Tarabanova, A.E., Papynov, E.K. et al. Hybrid Microwave Solid-Phase Synthesis of Wollastonite Based on Natural Renewable Raw Materials. Russ. J. Inorg. Chem. 67, 1400–1407 (2022). https://doi.org/10.1134/S0036023622090121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622090121