Abstract
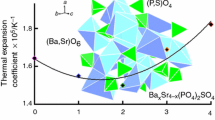
The phosphate–sulfate synthesis approach preventing the elimination of sulfur in the process of synthesis has been tested for NaBa6Zr(PO4)5SO4 as an example. The phase formation and thermal stability of phosphate–sulfate have been studied by X-ray diffraction and DTA-TG. The NaBa6Zr(PO4)5SO4 structure (space group I\(\overline 4 \)3d, a = 10.5449(3) Å, V = 1172.54(5) Å3, Z = 4) allied to the eulytite mineral has been refined by the Rietveld method. The structure is formed by wavy chains of edge-sharing (Na,Ba,Zr)O6-octahedra and (P,S)O4-tetrahedra sharing apices with the octahedra. Using thermal X-ray diffraction, it has been established that phosphate–sulfate is a strongly expanding material (αа = αb = αc = 13.3 × 10–6°C–1).






Similar content being viewed by others
REFERENCES
M. Ishii, K. Harada, N. Senguttuvan, et al., J. Cryst. Growth 205, 191 (1999). https://doi.org/10.1016/S0022-0248(99)00232-8
B. Onderka, Thermochim. Acta 601, 68 (2015). https://doi.org/10.1016/j.tca.2014.12.021
T. I. Milenov, P. M. Rafailov, R. Petrova, et al., Mater. Sci. Eng. 138, 35 (2007). https://doi.org/10.1016/j.mseb.2007.01.001
P. Yu, L. Su, H. Zhao, and J. Xu, J. Lumin. 154, 520 (2014). https://doi.org/10.1016/j.jlumin.2014.06.005
El H. Arbib, B. Elouadi, J. P. Chaminade, and J. Darriet, Mater. Res. Bull. 35, 761 (2000). https://doi.org/10.1016/S0025-5408(00)00270-1
P. P. Sahoo, E. Gaudin, J. Darriet, and Row T. N. Guru, Mater. Res. Bull. 44, 812 (2009). https://doi.org/10.1016/j.materresbull.2008.09.022
D. J. Segal, R. P. Santoro, and R. E. Newham, Z. Kristallogr. 123, 73 (1966). https://doi.org/10.1524/zkri.1966.123.16.73
P. Abhilash, M. T. Sebastian, and K. P. Surendran, J. Eur. Ceram. Soc. 36, 1939 (2016). https://doi.org/10.1016/j.jeurceramsoc.2016.02.019
H. F. Folkerts, J. Zuidema, and G. Blasse, Chem. Phys. Lett. 249, 59 (1996). https://doi.org/10.1016/0009-2614(95)01363-6
Z. Zhang, P. Shen, Ya. Wu, et al., Opt. Mater. 37, 866 (2014). https://doi.org/10.1016/j.optmat.2014.05.029
X. Chen, Z. Gong, Q. Wan, et al., Opt. Mater. 44, 48 (2015). https://doi.org/10.1016/j.optmat.2015.02.029
H. M. Rietveld, Acta Crystallogr. 22, 151 (1967).
Y. I. Kim and F. Izumi, J. Ceram. Soc. Jpn. 102, 401 (1994). https://doi.org/10.2109/jcersj.102.401
F. Izumi, The Rietveld Method (Oxford Univ. Press, New York, 1993).
J. Barbier, Eur. J. Solid State Inorg. Chem. 31, 163 (1994).
A. V. Knyazev, M. E. Komshina, A. V. Zhidkov, et al., Russ. J. Inorg. Chem. 58, 1172 (2013).
El H. Arbib, J.-P. Chaminade, J. Darriet, and B. Elouadi, Solid State Sci. 2, 243 (2000). https://doi.org/10.1016/S1293-2558(00)00132-1
R. S. Bubnova, M. G. Krzhizhanovskaya, and S. K. Filatov, The Manual on Thermal X-ray Diffraction Analysis of Polycrystals, Part 1: Experiments and Data Interpretation (St.-Petersburg Univ., St.-Petersburg, 2011).
V. I. Pet’kov, A. S. Dmitrienko, and A. I. Bokov, J. Therm. Anal. Calorim. 133, 199 (2018). https://doi.org/10.1007/s10973-017-6676-7
B. G. Vats, R. Phatak, K. Krishnan, et al., J. Alloys Compd. 690, 561 (2017). https://doi.org/10.1016/j.jallcom.2016.08.122
ACKNOWLEDGMENTS
The equipment of the Shared Facilities Center “new Materials and Resource-Saving Technologies” of the Lobachevskii National Research State University was used in this work.
Funding
This work was financially supported by the Russian Foundation for Basic Research (project no. 18-29-12063) with the use of the equipment of the Shared Facilities Center “New Materials and Resource-Saving Technologies” of the Lobachevskii National Research State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Pet’kov, V.I., Bokov, A.I., Asabina, E.A. et al. Synthesis, Crystal Structure, and Thermal Expansion of Sodium Barium Zirconium Phosphate–Sulfate. Russ. J. Inorg. Chem. 64, 1354–1358 (2019). https://doi.org/10.1134/S0036023619110159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619110159