Abstract
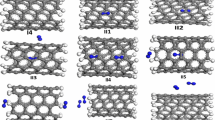
First-principles calculations based on density functional theory (DFT) are used to study the chemisorption properties of one, two, and four hydrogen atoms on the zigzag and armchair single-walled InN nanotubes (InNNTs).The results indicate that the H atom is strongly bounded to the exterior wall of (4, 4) InNNTs compared with the (7, 0) InNNTs, while the chemisorption energies corresponding to the most stable configuration of H2 dissociation and a single H atom are found to be–3.85 and–3.26 eV, respectively. Furthermore, the effect of the hydrogen storage on the geometries and electronic properties of related InN nanotubes were also discussed. The computed density of states (DOS) indicates that the energy gap of the zigzag and armchair InN nanotubes on hydrogen adsorptions are significantly decreased which can increase the electrical conductance of the tubes. Therefore, InN nanotubes due to the high binding energy can be used for hydrogen storage.
Similar content being viewed by others
References
N. Mohammad and H. Morkoc, Prog. Quantum Electron. 20, 361 (1996).
I. Vurgaftman and J. R. Meyer, J. Appl. Phys. 94, 3675 (2003).
L. Yin, Y. Bando, D. Golberg, and M. Li, Adv. Mater. 16, 1833 (2004).
K. Sardar, F. L. Deepak, A. Govindaraj, et al., Small 1, 91 (2005).
N. C. Chen, P. H. Chang, Y. N. Wang, et al., Appl. Phys. Lett. 87, 212111 (2005).
F. Zhang, Q. Wu, Y. Zhang, et al., Appl. Surf. Sci. 258, 9701 (2012).
O. Ambacher, M. S. Brandt, R. Dimitrov, et al., J. Vacuum Sci. Technol. B 14, 3532 (1996).
F. Ye, X. M. Cai, X. M. Wang, et al., Acta Phys. Sin. 56, 2342 (2007).
N. Ohba, K. Miwa, N. Nagasako, and A. Fukumoto, Phys. Rev. B 63, 115207 (2001).
Z. Qian, S. Hou, J. Zhang, et al., Physica E: Low-Dimensional Syst. Nanostruct. 30, 81 (2005).
Q. Wang, Q. Sun, P. Jena, and Y. Kawazoe, ACS Nano 3, 621 (2009).
M. T. Baei, A. Ahmadi Peyghan, and Z. Bagheri, Compt. Rend. Chim. 16, 302 (2013).
R. Ma, Y. Bando, H. W. Zhu, et al., J. Am. Chem. Soc. 124, 7672 (2002).
S. H. Lim and J. Lin, Chem. Phys. Lett. 466, 197 (2008).
C. Liu, Y. Y. Fan, M. Liu, et al., Science 286, 1127 (1999).
G.-X. Chen, Y. Zhang, D.-D. Wang, and J.-M. Zhang, J. Mol. Struct. (THEOCHEM) 956, 77 (2010).
M. Schmidt, K. Baldridge, J. Boatz, et al., J. Comput. Chem. 14, 1347 (1993).
A. Soltani, M. Ramezani Taghartapeh, E. Tazikeh Lemeski, et al., Superlattices Microstruct. 58, 178 (2013).
A. Soltani, N. Ahmadian, A. Amirazami, et al., Appl. Surf. Sci. 261, 262 (2012).
A. Soltani, N. Ahmadian, Y. Kanani, et al., Appl. Surf. Sci. 258, 9536 (2012).
A. Ahmadi Peyghan, M. T. Baei, M. Moghimi, and S. Hashemian, Comput. Theor. Chem. 997, 63 (2012).
A. Ahmadi, N. L. Hadipour, M. Kamfiroozi, and Z. Bagheri, Sens. Actuators B: Chem. 161, 1025 (2012).
S. S. Han, J. K. Kang, H. M. Lee, et al., J. Chem. Phys. 123, 114704 (2005).
Q. Sun, P. Jena, Q. Wang, and M. Marquez, J. Am. Chem. Soc. 128, 9741 (2006).
J. S. Arellano, L. M. Molina, A. Rubio, and J. A. Alonso, J. Chem. Phys. 112, 8114 (2000).
Y. Okamoto and Y. Miyamoto, J. Phys. Chem. B 105, 3470 (2001).
J. S. Arellano, L. M. Molina, A. Rubio, et al., J. Chem. Phys. 117, 2281 (2002).
Z. Bagheri and A. Ahmadi Peyghan, Comput. Theor. Chem. 1008, 20 (2013).
J. Beheshtian, H. Soleymanabadi, M. Kamfiroozi, and A. Ahmadi, J. Mol. Modeling 18, 2343 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Baei, M.T., Lemeski, E.T. & Soltani, A. A density-functional theory of hydrogen adsorption on indium nitride nanotubes. Russ. J. Inorg. Chem. 62, 325–335 (2017). https://doi.org/10.1134/S0036023617030044
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023617030044