Abstract
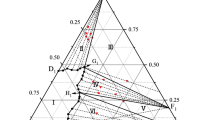
The equilibrium solubility of the quaternary system TbCl3-CdCl2-HCl(7.92 mas. %)-H2O was determined at 298K and the corresponding equilibrium diagram was constructed. The quaternary system is complicated by the three equilibrium solid phases: CdCl2 · H2O, TbCd4Cl11 · 14H2O (4: 1 type), and TbCl3 · 6H2O, of which the new compound Cd4TbCl11 · 14H2O was found to be congruently soluble in the system. The new compound obtained was identified and characterized by the method of X-ray diffraction and the thermal analysis methods of thermogravimetry-differential thermogravimetry (TG-DTG).
Similar content being viewed by others
References
Li Yahong, Ran Xinquan, and Chen Peiheng, J. Rare Earths 15, 113 (1997).
Jiao Huan, Wang Hui, Qan Xinquan, and Chen Peiheng, Acta Chim. Sin. 56, 854 (1998).
Li Yahong, Ran Xinquan, and Chen Peiheng, Chem. Pap. 52, 211 (1998).
Li Yahong, Ran Xinquan, and Chen Peiheng, Zh. Neorg. Khim. 44, 1207 (1999).
Wang Hui, Duan Jinxia, Ran Xinquan, and Gao Shiyang, Chin. J. Chem. 22, 1129 (2004).
Wang Hui, Duan Jinxia, Ran Xinquan, and Gao Shiyang, Chin. J. Chem. 20, 904 (2002).
Wang Hui, Duan Jinxia, Ran Xinquan, and Gao Shiyang, J. Chem. Thermodyn. 34, 1495 (2002).
Wang Hong, Qan Xinquan, and Chen Peiheng, Acta Chim. Sin. 52, 789 (1994).
Qiao Zhanping, Zhuo Lihong, Zhang Shushen, and Wang Hui, Chin. J. Inorg. Chem. 22, 1545 (2006).
Qiao Zhanping, Zhuo Lihong, and Wang Hui, Chin. J. Inorg. Chem. 20, 929 (2004).
Qiao Zhanping, Zhuo Lihong, Guo Yingchen, and Wang Hui, Acta Phys.-Chim. Sin. 21, 128 (2005).
Li Li, Wang Hui, Xia Shuping, Hu Mancheng, and Gao Shiyang, Chin. J. Inorg. Chem. 19, 201 (2003).
Qiao Zhanpinp, Zhuo Lihong, Chen Xin, and Wang Hui, Chin. J. Inorg. Chem. 22, 856 (2006).
Wang Hui, Li Li, Ran Xinquan, Wang Xiapeng, and Gao Shiyang, J. Chem. Eng. Data 51, 1541 (2006).
Qiao Zhanping, Zhuo Lihong, Guo Yingchen, and Wang Hui, Chin. J. Inorg. Chem. 21, 1667 (2005).
Qiao Zhanping, Zhuo Lihong, Guo Yingchen, and Wang Hui, Acta Phys.-Chim. Sin. 21, 1249 (2005).
Qiao Zhanping, Zhuo Lihong, Guo Yingchen, and Wang Hui, Acta Phys.-Chim. Sin. 22, 616 (2006).
Chen Yunsheng, Analysis of Physical Chemistry (Higher Education Press, Beijing, 1988).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, Z., Zhuo, L., Chen, X. et al. Phase Equilibrium of the System TbCl3-CdCl2-HCl(7.92 wt %)-H2O at 298.15 ± 0.1 K 1. Russ. J. Inorg. Chem. 53, 446–449 (2008). https://doi.org/10.1134/S0036023608030194
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023608030194