Abstract
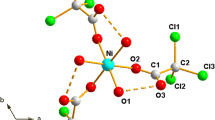
Complex formation in the M(HCOO)2-CS(NH2)2-H2O (M = Mg, Mn, Cd) systems at 25°C is studied using the isothermal solubility method. In the Cd(HCOO)2-CS(NH2)2-H2O system, a congruently dissolving compound Cd(HCOO)2 · 2CS(NH2)2 is found and characterized by X-ray powder diffraction and IR spectroscopy. The Mg(HCOO)2-CS(NH2)2-H2O and Mn(HCOO)2-CS(NH2)2-H2O systems are eutonics at this temperature. Data on carbamide and thiocarbamide complexes of divalent metal formates are systematized.
Similar content being viewed by others
References
M. Kydynov, Reactions of Thiourea and Urea with Mineral Salts (Ilim, Frunze, 1965) [in Russian].
P. I. Protsenko, A. G. Glinina, and G. P. Protsenko, Zh. Neorg. Khim. 16(12), 3305 (1971).
L. D. Dremyatskaya, N. B. Lyubimova, and S. D. Beskov, Zh. Fiz. Khim. 18(4), 850 (1969).
R. Petrova, S. Bakardjieva, and T. Todorov, Z. Kristallogr. 215(2), 118 (2000).
Mary P.A. Angeli and S. Danuskodi, Cryst. Res. Technol. 36(11), 1231 (2001).
M. Oussaide, P. Becker, and C. Carabatos-Nedelec, Phys. Status Solidi B 207(2), 499 (1998).
H. O. Marcy, L. F. Warren, M. S. Web, et al., Appl. Opt. 31(24), 5051 (1992).
V. Venkataramanan, C. K. Subramanian, and H. L. G. Bhat, Appl. Phys. 77, 6049 (1995).
J. M. Alia, H. G. M. Edwards, and M. D. Stoev, Spectrochim. Acta A 55(12), 2423 (1999).
G. V. Romanenko, L. I. Myachina, and S. V. Larionov, Zh. Strukt. Khim. 42(2), 387 (2001).
M. Nardelli, G. G. Fava, and P. Boldrini, Gazz. Chim. Ital. 92(12), 1392 (1962).
M. Nardelli, G. G. Fava, and P. Boldrini, Acta Crystallogr. 18(4), 618 (1965).
V. Z. Vassileva and P. P. Petrova, Croat. Chem. Acta 78(2), 295 (2005).
K. Gyeryova, V. Balek, and V. Zelenak, Thermochim. Acta 234, 221 (1994).
S. Duishekeeva, S. Zhumalieva, and K. R. Rysmendeev, Tr. Kirg. Univ., Ser. Khim. Nauk, No. 3, Part 1, 63 (1975)
F. W. Ashton, D. F. Houston, and C. P. Saylor, J. Res. Nat. Bur. Stand. 11, 233 (1933).
Chr. Balarew, D. Stoilova, and V. Vassileva, Commun. Depart. Chem. Bulg. Acad. Sci. 14(1), 57 (1981).
C. C. Balarew, D. G. Stoilova, and V. Z. Vassileva, Compt. Rend. Acad. Bulg. Sci. 35(7), 933 (1982).
G. de With, S. Harkema, and P. G. J. van Hummel, Acta Crystallogr. 32(7), 1980 (1976).
G. Weber, Z. Kristallogr. 158(3–4), 315 (1982).
M. L. Post and J. Trotter, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 30(9), 315 (1974).
V. G. Khlopin, Selected Works (Nauka, Leningrad, 1957), Vol. 1, p. 162 [in Russian].
G. Schwarzenbach and H. Flaschka, Die komplexometrische Titration (Ferdinand Euke, Stuttgart, 1965; Khimiya, Moscow, 1970).
I. Lysyj and J. E. Zarembo, Anal. Chem. 18(3), 428 (1958).
F. A. H. Schreinemakers, Z. Phys. Chem. 11, 76 (1906).
M. M. Elcome and J. C. Taylor, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr. 24(4), 410 (1968).
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds (Wiley, New York, 1986), p. 269.
A. Yamaguchi, R. B. Penland, S. Mizushima, et al., J. Am. Chem. Soc. 80(3), 527 (1958).
K. Swaminathan and H. M. N. Irving, J. Inorg. Nucl. Chem. 26(7), 1291 (1964).
J. D. Donaldson, J. F. Knifton, and S. D. Ross, Spectrochim. Acta 20(5), 847 (1964).
G. D. Tewari, V. P. Tayal, D. P. Khandelwal, and H. D. Bist, Appl. Spectrosc. 36(4), 847 (1982).
A. M. Heyns, J. Mol. Struct. 127(1), 9 (1985).
K. Yamagata, Y. Sayto, T. Abe, and M. Hashimoto, J. Phys. Soc. Jpn. 58(10), 3865 (1989).
K. Yamagata, Y. Sayto, T. Abe, and M. Hashimoto, J. Phys. Soc. Jpn. 58(2), 752 (1989).
S. Abraham and G. Aruldhas, Spectrochim. Acta 51A(1), 79 (1995).
K. A. Nadzharyan, I. M. Kaganskii, E. V. Stamikosto, and L. Eraizer, Zh. Neorg. Khim. 34(8), 2152 (1998).
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.Z. Vasileva, P.P. Petrova, 2006, published in Zhumal Neorganicheskoi Khimii, 2006, Vol. 51, No. 5, pp. 880–884.
Rights and permissions
About this article
Cite this article
Vasileva, V.Z., Petrova, P.P. Reactions in the M(HCOO)2-CS(NH2)2-H2O (M = Mg, Mn, Cd) systems at 25°C. Russ. J. Inorg. Chem. 51, 814–818 (2006). https://doi.org/10.1134/S0036023606050226
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0036023606050226