Abstract
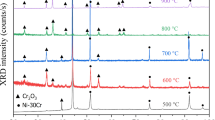
In calculations, not only the Gibbs formation energy of nickel and chromium oxides and thermodynamic activities of the alloy components, but also the Gibbs surface energy of a Ni-Cr alloy is taken into account. The method is based on the adsorption model of the alloy oxidation, according to which the adsorption of an alloy component with the smaller surface energy, namely, nickel, at the alloy-oxide film interface boundary shifts the dynamic equilibrium between the alloy and oxide-film components toward the formation of NiO. For the oxidation of Ni-Cr alloys, which are solid solutions with an fcc lattice, at 1123 to 1473 K, concentration boundaries of the chromium content in the alloy are calculated for the case of the prevailing formation of Cr2O3 oxide, which determines the high heat resistance of Ni-Cr alloys.
Similar content being viewed by others
References
Mrovec, S. and Werber, T., Nowoczesne materialy zaroodporne Wydawnictwa naykowo-techniezne, Waszawa, 1982.
Birks, N. and Meier, G.H., Introduction to High Temperature Oxidation of Metals, Edward Arnolg, 1983.
Chemical Thermodynamics in Industry, Barry, T.T., Ed., Oxford, 1985.
Kubashevski, O. and Alcock, C.B., Metallurgical Thermochemistry, London: Pergamon Press, 1968.
Wagner, C.J., J. Electrochem. Soc., 1952, vol. 99, p. 369; 1956, vol. 103, p. 627.
Chuang, Y.Y. and Chang, Y.A., Z. Metallkunde, 1986, vol. 77, p. 460.
Zhukhovitskii, A.A., Zh. Fiz. Khimii, 1944, vol. 18, no. 5–6, p. 214.
Andreev, Yu.Ya., Zh. Fiz. Khimii, 2002, vol. 76, no. 2, p. 338.
Andreev, Yu.Ya., Zh. Fiz. Khimii, 1998, vol. 72, no. 3, p. 529.
Andreev, Yu.Ya., Electrochim. Acta, 1998, vol. 43, p. 2627.
Andreev, Yu.Ya., Zh. Fiz. Khimii, 2000, vol. 74, no. 5, p. 913.
Andreev, Yu.Ya. and Kutyrev, A.E., Zh. Fiz. Khimii, 2001, vol. 75, no. 4, p. 689.
Andreev, Yu.Ya., Materialovedenie, 2004, no. 9, p. 2.
Andreev, Yu.Ya., Zh. Fiz. Khimii, 2005, vol. 79, no. 2, p. 239.
Bokshtein, B.S., Diffuziya v metallakh (Diffusion in Metals), Moscow: Metallurgiya, 1978.
Vitos, L., Ruban, A.V., and Skriver, H.L., Surf. Sci., 1998, vol. 411, p. 186.
Wicks, C.E. and Block, F.E., Thermodynamic Properties of 65 Elements, Their Oxides, Halides, Carbides, and Nitrides, USA Department of the Interior, 1960.
Croll, E. and Wallwork, G., Oxid. Met., 1969, vol. 1, p. 55.
Author information
Authors and Affiliations
Additional information
Original Russian Text © Yu.Ya. Andreev, A.A. Shumkin, 2006, published in Zashchita Metallov, 2006, Vol. 42, No. 3, pp. 239–244.
Rights and permissions
About this article
Cite this article
Andreev, Y.Y., Shumkin, A.A. A new theoretical approach to the thermodynamic calculation of high-temperature oxidation of Ni-Cr alloys. Prot Met 42, 221–226 (2006). https://doi.org/10.1134/S0033173206030039
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0033173206030039