Abstract
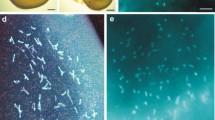
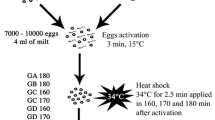
Viable prelarvae were obtained for the first time as a result of insemination of eggs of the kaluga Acipenser dauricus with UV-inactivated sperm of the sterlet A. ruthenus. These normally developed prelarvae without any signs of the haploid syndrome carried two maternal alleles at each examined microsatellite locus; they did not have sterlet paternal alleles. The viability of haploid gynogenetic prelarvae is explained by the low level of diploidization of the kaluga genome, which is confirmed by the presence of multivalent configurations (mainly quadrivalents) in prophase I of meiosis in kaluga (with ~260 chromosomes). The results correspond to the extremely low rate of molecular and chromosomal evolution noted for all sturgeons.



Similar content being viewed by others
REFERENCES
Andreyushkova, D.A., Makunin, A.I., Beklemisheva, V.R. et al., Next generation sequencing of chromosome−specific libraries sheds light on genome evolution in paleotetraploid sterlet (Acipenser ruthenus), Genes, 2017, vol. 8, no. 11, pp. 1–12. https://doi.org/10.3390/genes8110318
Arai, K., Matsubara, K., and Suzuki, R., Chromosomes and developmental potential of progeny of spontaneous tetraploid loach Misgurnus anguillicaudatus, Nipp. Suisan Gakkaishi, 1991, vol. 57, no. 12, pp. 2173–2178. https://doi.org/10.2331/suisan.57.2173
Arai, K., Matsubara, K., and Suzuki, R., Production of polyploids and viable gynogens using spontaneously occurring tetraploid loach, Misgurnus anguillicaudatus, Aquaculture, 1993, vol. 117, no. 3–4, pp. 227–235. https://doi.org/10.1016/0044?8486(93)90322?P
Arefjev, V.A., Polykaryogrammic analysis of the ship Acipenser nudiventris Lovetsky (Acipenseridae, Chondrostei), J. Ichthyol., 1983, vol. 23, pp. 26–35.
Badrtdinov, O.A., Kovalev, K.V., Lebedeva, E.B., et al., Entirely male gynogenetic offspring of Acipenser stellatus (Pisces, Acipenseridae), Dokl. Biol. Sci., 2008, vol. 423, no. 1, pp. 392–394. https://doi.org/10.1134/s0012496608060070
Barmintseva, A.E. and Mugue, N.S., The use of microsatellite loci for identification of sturgeon species (Acipenseridae) and hybrid forms, Russ. J. Genet., 2013, vol. 49, no. 9, pp. 950–961. https://doi.org/10.1134/S1022795413090032
Bemis, W.E., Findeis, E.K., and Grande, L., An overview of Acipenseriformes, Environm. Biol. Fish., 1997, vol. 48, pp. 25–71.
Berrebi, P., Cattaneo-Berrebi, G., and Le Brun, N., Natural hybridization of two species of tetraploid barbels: Barbus meridionalis and Barbus barbus (Osteichthyes, Cyprinidae) in southern France, Biol. J. Linn. Soc., 1993, vol. 48, pp. 319–333.
Cherfas, N.B., Rothbard, S., Hulata G., and Kozinsky, O., Spontaneous diploidization of maternal chromosome set in ornamental (koi) carp, Cyprinus carpio L., J. Appl. Ichthyol., 1991, vol. 7, pp. 72–77.
Cherfas, N.B., Gomelsky, B., Ben-Dom, M.N., and Hulata, G., Evidence for the heritable nature of spontaneous diploidization in common carp, Cyprinus carpio L., eggs, Aquacult. Res., 1995, vol. 26, pp. 289–292.
Chourrout, D., Gynogenesis caused by ultraviolet irradiation of salmonid sperm, J. Exp. Zool., 1982, vol. 223, pp. 175–181.
Chourrout, D., Chevassus, B., and Herioux, F., Analysis of an Hertwig effect in the rainbow trout (Salmo gairdneri Richardson) after fertilization with y-irradiated sperm, Reprod. Nutr. Devel., 1980, vol. 20, pp. 719–726.
De la Herrán, R., Fontana, F., Lanfredi, M., et al., Slow rates of evolution and sequence homogenization in an ancient satellite DNA family of sturgeons, Mol. Biol. Evol., 2001, vol. 18, no. 1, pp. 432–436. https://doi.org/10.1093/oxfordjournals.molbev.a003820
Dingerkus, G. and Howell, W.M., Karyotypic analysis and evidence of tetraploidy in the North American paddlefish, Polyodon spathula, Science, 1976, vol. 194, no. 4267, pp. 842–844.
Du, K., Stöck, M., Kneitz, S., et al., The sterlet sturgeon genome sequence and the mechanisms of segmental rediploidization, Nat. Ecol. Evol., 2020, vol. 4, pp. 841–852. https://doi.org/10.1038/s41559?020?1166?xoi.org/10.1038/s41559?020?1166?x
Flajšhans, M., Kvasnicka, P., and Ráb, P., Genetic studies in tench (Tinca tinca L.): high incidence of spontaneous triploidy, Aquaculture, 1993, vol. 110, pp. 243–248. https://doi.org/10.1016/0044?8486(93)90372?6
Fontana, F., Tagliavini, J., and Congiu, L., Sturgeon genetics and cytogenetics: recent advancements and perspectives, Genetica, 2001, vol. 111, pp. 359–373.
Fontana, F., Congiu, L., Mudrak, V.A., Quattro, J.M., et al., Evidence of hexaploid karyotype in shortnose sturgeon, Genome, 2008, vol. 51, no. 2, pp. 113–119.https://doi.org/10.1139/g07?112
Fopp-Bayat, D., Spontaneous gynogenesis in Siberian sturgeon Acipenser baeri Brandt, Aquacult. Res., 2007, vol. 38, pp. 776–779. https://doi.org/10.1111/j.1365?2109.2007.01739.x
Fopp-Bayat, D. and Woznicki, P., Spontaneous and induced gynogenesis in sterlet Acipenser ruthenus Brandt, Caryologia, 2007, vol. 60, no. 4, pp. 315–318. https://doi.org/10.1080/00087114.2007.10797953
Gardiner, B.G., Sturgeons as living fossils, in Living Fossils, Eldredge, N. and Stanley, S.M., Eds., New York: Springer Verlag, 1984, pp. 148–152.
Havelka, M., Hulák, M., and Bailie, D.A., Extensive genome duplications in sturgeons: new evidence from microsatellite data, J. Appl. Ichthyol., 2013, vol. 29, pp. 704–708. https://doi.org/10.1111/jai.12224
Havelka, M., Bytyutskyy, D., Symonovẚ, R., et al., The second highest chromosome count among vertebrates is observed in cultured sturgeon and is associated with genome plasticity, Gen. Sel. Evol., 2016, vol. 48, no. 1, pp. 1–9.https://doi.org/10.1186/s12711?016?0194?0
Hilton, E.J., Kovalchuk, O., Podoplelova, N., Sturgeon (Acipenseridae) from the Late Miocene of Ukraine, with a discussion of materials associated with Widhalm’s (1886) nomen nudum, † Acipenser euhuso, Zootaxa, 2021, vol. 5057, no. 3, pp. 85–101. https://doi.org/10.11646/zootaxa.5057.3.4
Ivanova, N.V. and Hebert, P.D.N., An inexpensive, automation friendly protocol for recovering high quality DNA, Mol. Ecol. Notes, 2006, vol. 6, pp. 998–1002.https://doi.org/10.1111/j.1471?8286.2006.01428.x
Kochakpour, N., Immunofluorescent microscopic study of meiosis in zebrafish, Meth. Mol. Biol., 2009, vol. 558, pp. 251–260. https://doi.org/10.1007/978?1?60761?103?5_15
Komen, J., Duynhouwer, J., Richter, C.J.J., and Huisman, E.A., Gynogenesis in common carp (Cyprinus carpio L.). I. Effects of genetic manipulation of sexual products and incubation conditions of eggs, Aquaculture, 1988, vol. 69, pp. 227–239.
Krieger, J. and Fuerst, P.A., Evidence for a slowed rate of molecular evolution in the order Acipenseriformes, Mol. Biol. Evol., 2002, vol. 19, no. 6, pp. 891–897.https://doi.org/10.1093/oxfordjournals.molbev.a004146
Krieger, J., Hett, A.K., Fuerst, P.A., et al., The molecular phylogeny of the order Acipenseriformes revisited, J. Appl. Ichthyol., 2008, vol. 24, no. s1, pp. 36–45. https://doi.org/10.1111/j.1439?0426.2008.01088.x
Kusunoki, T., Arai, K., and Suzuki R., Production of viable gynogens without chromosome duplication in the spinous loach Cobitis biwae, Aquaculture, 1994, vol. 119, no. 1, pp. 11–23. https://doi.org/10.1016/0044?8486(94)90440?5
Li, Y.-J., Yu, Zh., Zhang, M.-Zh., et al., The origin of natural tetraploid loach Misgurnus anguillicaudatus (Teleostei: Cobitidae) inferred from meiotic chromosome configurations, Genetica, 2011, vol. 139, no. 6, pp. 805–811. https://doi.org/10.1007/s10709?011?9585?x
Ludwig, A., Belfiore, N.M., Pitra, C., et al., Genome duplication events and functional reduction of ploidy levels in sturgeon (Acipenser, Huso and Scaphirhynchus), Genetics, 2001, vol. 158, no. 3, pp. 1203–1215. https://doi.org/10.1093/genetics/158.3.1203
McQuown, E., Sloss, B.L., Sheehan, R.J., et al., Microsatellite analysis of genetic variation in sturgeon: new primer sequences for Scaphirhynchus and Acipenser, Trans. Am. Fish. Soc., 2000, vol. 129, no. 6, pp. 1380–1388. https://doi.org/10.1577/1548?8659(2000)129<1380:MAOGVI>2.0.CO;2
Moens, P.B., Zebrafish: chiasmata and interference, Genome, 2006, vol. 49, pp. 205–208. https://doi.org/10.1139/g06-021
Nagy, A., Rajki, K., Harvath, L., and Csanyi, V., Investigation on carp, Cyprinus carpio L. gynogenesis, J. Fish Biol., 1978, vol. 13, pp. 215–224.
Nelson, J.S., Fishes of the World, New Jersey: Wiley, 2006.
Onozato, H., The “Hertwig effect” and gynogenesis in chum salmon Oncorhynchus keta eggs fertilized with (60)Co gamma-ray irradiated milt, Bull. Jpn. Soc. Sci. Fish., 1982, vol. 48, pp. 1237–l244.
Onozato, H. and Yamaha, E., Induction of gynogenesis with ultraviolet rays in four species of salmoniforms, Bull. Jpn. Soc. Sci. Fish., 1983, vol. 49, pp. 693–699.
Peng, Z., Ludwig, A., Wang, D., et al., Age and biogeography of major clades in sturgeons and paddlefishes (Pisces: Acipenseriformes), Mol. Phyl. Evol., 2007, vol. 42, no. 3, pp. 854–862. https://doi.org/10.1016/j.ympev.2006.09.008
Pyatskowit, J.D., Krueger, C.C., Kincaid, H.L., and May, B., Inheritance of microsatellite loci in the polyploid lake sturgeon (Acipenser fulvescens), Genome, 2001, vol. 44, no. 2, pp. 185–191. https://doi.org/10.1139/g00?118
Rajkov, J., Shao, Zh., and Berrebi, P., Evolution of polyploidy and functional diploidization in sturgeons: microsatellite analysis in 10 sturgeon species, J. Heredity, 2014, vol. 105, no. 4, pp. 521–531. https://doi.org/10.1093/jhered/esu027
Recoubratsky, A.V., Grunina, A.S., Myuge, N.S., and Neyfakh, A.A., Production of androgenetic nucleocytoplasmic hybrids in sturgeon fish, Russ. J. Devel. Biol., 1998, vol. 29, pp. 224–229.
Recoubratsky, A.V., Grunina, A.S., Barmintsev, V.A., et al., Meiotic gynogenesis in the stellate and Russian sturgeons and sterlet, J. Heredity, 2003, vol. 34, pp. 92–101. https://doi.org/10.1023/A:1023396213126
Robles, F., de la Herran, R., Ludwig, A. et al., Genomic organization and evolution of the 5S ribosomal DNA in the ancient fish sturgeon, Genome, 2005, vol. 48, pp. 18–28. https://doi.org/10.1139/g04?077
Rodzen, J.A., and May, B., Inheritance of microsatellite loci in the white sturgeon (Acipenser transmontanus), Genome, 2002, vol. 45, no. 6, pp. 1064–1076. https://doi.org/10.1139/g02?083
Romanenko, S.A., Biltueva, L.S., Serdyukova, N.A., et al., Segmental paleotetraploidy revealed in sterlet (Acipenser ruthenus) genome by chromosome painting, Mol. Cytogenet., 2015, vol. 8, no. 1, pp. 1–13. https://doi.org/10.1186/s13039?015?0194?8
Safronov, A.S., Rachek, E.I., Zuevsky, S.E., et al., Results of comparative cultivation of kaluga, amur sturgeon and reciprocal hybrids between them with using of various technologies, Izv. Tikhook. Inst. Rybn. Khoz. Okeanogr., 2021, vol. 201, no. 4, pp. 923–936. https://doi.org/10.26428/1606?9919?2021?201?923?936
Saitoh, K., Mitotic and meiotic analyses of the “large race” of Cobitis striata, a polyploid spined loach of hybrid origin, Folia Biol. (Krakow), 2003, vol. 5l, Supp1., pp. 101–105.
Simanovsky, S.A., Matveevsky, S.N., Rachek, E.I., et al., Analysis of meiotic chromosomes in some species and hybrids of sturgeons in the context of polyploid evolution, Proc. VIII Sci.-Pract. Conference “Genetics − a fundamental basis for innovations in medicine and breeding”, Rostov-on-Don; Taganrog: Southern Federal University Press, 2019, pp. 76–77.
Suzuki, R., Oshiro, T., and Nakanishi, T., Survival, growth and fertility of gynogenetic diploids induced in the cyprinid loach Misgurnus anguillicaudatus, Aquaculture, 1985, vol. 48, pp. 45–55.
Tang, Q., Freyhof, J., Xiong, B., and Liu, H., Multiple invasions of Europe by East Asian cobitid loaches (Teleostei: Cobitidae), Hydrobiologia, 2008, vol. 605, pp. 17–28. https://doi.org/10.1007/s10750?008?9296?1
Van Eenennaam, A.L., Murray, J.D., and Medrano, J.F., Synaptonemal complex analysis in spermatocytes of white sturgeon, Acipenser transmontanus Richardson (Pisces, Acipenseridae), a fish with a very high chromosome number, Genome, 1998, vol. 41, no. 1, pp. 51–61. https://doi.org/10.1139/g97-101
Vasil’ev, V.P., Evolyutsionnaya kariologiya ryb (Evolutionary Karyology of Fish), Moscow: Nauka, 1985.
Vasil’ev, V.P., Mechanisms of polyploid evolution in fish: polyploidy in sturgeons, Biology, Conservation and Sustainable Development of Sturgeons. Fish and Fisheries Series, vol. 29, Dordrecht: Springer, 2009, pp. 97–117.
Vasil’ev, V.P. and Vasil’eva, E.D., Polyploid evolution and functional genome diploidization in sturgeons, The 10th Indo-Pacific Fish Conference, Book of Abstract, Tahiti, CRIOBE, 2017, pp. 42. https://ipfc10.criobe.pf/cms/wp−content/uploads/2017/09/book−abstracts−ipfc10.pdf
Vasil’ev, V.P., Vasil’eva, E.D., Shedko, S.V., and Novomodny, G.V., Ploidy levels in the kaluga, Huso dauricus and Sakhalin sturgeon Acipenser mikadoi (Acipenseridae, Pisces), Dokl. Biol. Sci., 2009, vol. 426, pp. 228–231. https://doi.org/10.1134/s0012496609030119
Vasil’ev, V.P., Vasil’eva, E.D., Shedko, S.V., and Novomodny, G.V., How many times has polyploidization occurred during Acipenserid evolution? New data on the karyotypes of sturgeons (Acipenseridae, Actinopterygii) from the Russian Far East, J. Ichthyol., 2010, vol. 50, no 10, pp. 950−959. https://doi.org/10.1134/S0032945210100048
Vasil’ev, V.P., Rachek, E.I., Lebedeva, E.B., and Vasil’eva, E.D., The karyological study in backcross hybrids between the sterlet, Acipenser ruthenus, and kaluga, A. dauricus (Actinopterygii: Acipenseriformes: Acipenseridae): A. ruthenus x (A. ruthenus x A. dauricus) and A. dauricus x (A. ruthenus x A. dauricus), Acta Ichthyol. Piscat., 2014, vol. 44, no. 4, pp. 301–308. https://doi.org/10.3750/AIP2014.44.4.04
Vasil’ev, V.P., Medvedev, D.A., Rachek, E.I., et al., Can evolutionary diploid genome of tetraploid sturgeon species maintain the functional properties of the normal diploid genome?, Proc. Internat. Sci. Conference “Genetics of Populations: Progress and Perspectives”, Moscow: Vash Format, 2017, pp. 50–51.
Vasil’ev, V.P., Rachek, E.I., Amvrosov D.Yu., et al., Fertility of females of sturgeon hybrids obtained from species with different levels of ploidy (Acipenser ruthenus and A. dauricus) and their cloning, J. Appl. Ichthyol., 2021, vol. 37, no. 2, pp. 186–197. https://doi.org/10.1111/jai.14168
Welsh, A.B., Blumberg, M., and May, B., Identification of microsatellite loci in lake sturgeon, Acipenser fulvescens, and their variability in green sturgeon, A. medirostris, Mol. Ecol. Notes, 2003, vol. 3, pp. 47–55. https//doi.org/ https://doi.org/10.1046/j.1471?8286 .2003.00346.x
Wolfe, K.H., Yesterday’s polyploids and the mystery of diploidization, Nat. Rev. Genet., 2001, vol. 2, pp. 333–341. https://doi.org/10.1038/35072009
Wu, C., Ye, Y., and Chen, R., Genome manipulation in carp (Cyprinus carpio L.), Aquaculture, 1986, vol. 54, pp. 57–61.
ACKNOWLEDGMENTS
We are very grateful to D. Amvrosov and E. Rachek who provided us with materials from LRFBS for our experimental crosses and genetic analyzes, as well as for their help in organizing and conducting our research at LRFBS. The authors also thank the anonymous reviewers for their valuable comments and suggestions, which undoubtedly improved the manuscript.
Funding
Experimental crosses in LRFBS, as well as microsatellite and synaptonemal complex analyses were conducted in 2017 and 2018 with partial financial support of the Russian Fund of Basic Researches; further scientific investigations of Ekaterina Vasil’eva were carried within a State Project of Moscow State University no. 121032300105-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Vasil’ev, V.P., Simanovsky, S.A., Barmintseva, A.E. et al. Can the Ovum Genome of Tetraploid Sturgeon Species (Acipenseridae) Exhibit the Functional Properties of a Diploid Genome?. J. Ichthyol. 62, 1430–1438 (2022). https://doi.org/10.1134/S0032945222060303
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945222060303