Abstract
Diploid and triploid hybrid females of Cobitis as a rule produce unreduced eggs which mainly develop gynogenetically, but some of the eggs incorporate sperm genome and develop into triploids and tetraploids, respectively. Here, we observed for the first time the meiotic chromosomes in the germinal vesicles (GVs) of mature oocytes of three diploid C. taenia (2n = 48) and 20 allopolyploid females of Cobitis (18 triploid 3n = 74 and 2 tetraploid 4n = 99). The majority of GVs in diploid, triploid and tetraploid females contained 24, 74 and 96 or 99 bivalents, respectively. These results directly indicated premeiotic endomitosis as a mechanism underlying the formation of unreduced eggs in allopolyploid females of Cobitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyploids are relatively common among fish and they are usually well adapted (Comai 2005; Otto 2007). The hybrid pathway of polyploidization (allopolyploidy) is a potential mechanism of establishment of polyploid species and also leads to various modes of unisexual reproduction (parthenogenesis, gynogenesis or hybridogenesis) (Nabais et al. 2012). Unisexual polyploids can produce fertile unreduced gametes of various ploidy levels which can significantly contribute to polyploid formation (Gregory and Mable 2005). The formation of polyploids and subsequent diploidization are important aspects in long-term evolution and speciation (Comai 2005; Otto 2007).
Loaches of the genus Cobitis are small-sized, bottom-dwelling temperate fish represented by c. 70 species widely distributed in Europe and Asia (Kottelat 2012). Some of them evolved via hybridization and polyploidization, and then they parted from bisexual reproducing diploids acquiring unisexual reproductive mode such as hybridogenesis and gynogenesis in diploids and triploids (Saitoh et al. 2004), gynogenesis or bisexual reproduction in tetraploids (Boron 2003; Janko et al. 2007, 2012; Saitoh et al. 2010; Juchno et al. 2014).
Experimental studies revealed that triploid females of Cobitis from populations in Poland produced unreduced triploid eggs, which were gynogenetically activated by sperm of Cobitis taenia to develop into viable triploid progeny, but simultaneously some of the eggs were fertilized to provide less viable tetraploid progeny (Juchno and Boroń 2006; Juchno et al. 2014). The mechanisms responsible for unreduced gametogenesis have not been identified in polyploid Cobitis inhabiting Polish diploid–polyploid populations.
There are two major cytological mechanisms responsible for the formation of unreduced eggs in unisexual fish: a) premeiotic endomitosis and b) apomixis (Arai and Fujimoto 2013). The aim of our study is to answer the question which mechanism (a or b) is involved in unreduced oogenesis of triploid and tetraploid females of Cobitis. Here, we present, for the first time, the meiotic chromosome configurations in GVs of loaches of Cobitis with different ploidy levels.
Materials and methods
A total of 23 females were collected from diploid–polyploid populations: a) the Bug River (52°32′00″111N; 21°15′12″E), the Vistula River drainage, b) Kortówka River (53°45′43″N; 20°112 26′42″E), the Pregola River drainage (Baltic Sea basin), and c) as a reference Cobitis taenia (2n = 48) from an exclusively diploid population, Legińskie Lake (53°58′54″N; 21°08′20″E), by net and then transported alive to the laboratory. Different ploidy level of Cobitis loaches are not distinguished by external morphology, so their ploidy status and karyotype structure were identified postmortem by chromosome analysis. Among them, 18 triploids (3n = 74) and two tetraploids (4n = 99) were detected.
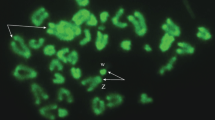
Cytological observations of germinal vesicles (GVs) were made according to the method described by Itono et al. (2006) and Morishima et al. (2008). The females were injected with ovopel (containing a GnRH analog and metoclopramide) and kept for 4-5 hours at 25 °C in an aquarium. Full-grown oocytes were isolated using tweezers from the ovary and then incubated in Ringers solution containing 17α-20β dihydroxy-4-pregnen-3-one (Sigma-Aldrich) at room temperature to induce in vitro final maturation. During the period from germinal vesicle migration (GVM) to immediately prior to germinal vesicle breakdown (GVBD), the oocytes were periodically collected and fixed in 5 % acetic acid, and the GV (Fig. 1a) was mechanically taken with fine forceps under a stereoscopic microscope and fixed with Carnoy’s solution. An isolated GV (Fig. 1b) was placed on a clean glass slide, air dried and then stained with DAPI (4′,6-diamidino-2-phenylindole) for 30 min. Meiotic chromosomes were observed under a Nikon Eclipse 90i fluorescence microscope equipped with ProgRes MFcool camera (Jenoptic) for capturing the DAPI-stained images. The images were processed using Lucia ver. 2.0 (Laboratory Imaging). Fish sampling and valid animal use protocols for experiments were performed with the permissions of the Polish Ministry of Environment (no. DLOPiK-op/ogiz-4200/V-5/5164/07/aj. and DOP-OZGiZ.6401.10.12.2011.ls) and the Local Ethics Committee of Poland (no. 37/2007, 20/01 and no. 04/2011).
Germinal vesicle of Cobitis. a Oocyte with germinal vesicle (GV); b isolated GV; c, d, e DAPI-stained germinal vesicle of the diploid oocyte at the diplotene showing 24 bivalents, triploid oocyte at the diplotene showing 74 bivalents, and tetraploid oocyte at the diakinesis showing 96 bivalents, respectively. Scale bars 150 μm (a–b), 10 μm (c–e)
Results
A total of 13, 42 and 8 GVs of Cobitis taenia, triploid and tetraploid females of Cobitis, respectively, were analyzed. In three diploid females of C. taenia (2n = 48), 11 out of 13 GVs observed clearly showed exactly 24 bivalents and the other two showed 23 bivalents possessing chiasmata (Fig. 1c) (Table 1). In GVs of triploid and tetraploid females also, the bivalents with chiasmata were visible. The majority of GVs isolated from the oocytes of triploids (3n = 74) contained 74 bivalents (Fig. 1d; Table 1). Most (60 %) of GVs of triploids gave a configuration including 74 bivalents, whereas 21 % of GVs showed 73 bivalents and other GVs contained 68 to 65 bivalents (Table 1). In two tetraploids (4n = 99), 96 or 99 bivalents were countable (Table 1). None of the analyzed triploid and tetraploid females exhibited univalents, trivalents or quadrivalents in diplotene oocytes.
Discussion
We have demonstrated that both triploid and tetraploid hybrid loaches of Cobitis apparently produced unreduced eggs by the cytological mechanisms of premeiotic endomitosis, because the number of bivalents was essentially the same as the number of chromosomes in the somatic cells. The present results strongly support previous histological observations of the gonad suggesting the presence of both the first and the second meiotic divisions in oocytes of polyploids of Cobitis (Lees and Saat 2003; Juchno et al. 2007). Therefore, we can rule out the mechanism of apomixis, because if it occurs, the first meiotic division would be skipped and the oocytes would undergo only the second division (Yamashita et al. 1993).
The unreduced gametogenesis by premeiotic endomitosis was already recognized in the clonal diploid pond loach Misgurnus anguillicaudatus (2n = 50) with hybrid origin (Itono et al. 2006) as well as in the triploid hybrids (3n = 75) between wild-type diploid (2n = 50) and natural tetraploid (4n = 100) (Zhang et al. 1998). However, other triploid hybrids between diploid and tetraploid in China exhibited meiotic configurations including bivalents, univalent, and infrequently trivalents (Li et al. 2015), and clone-origin triploids generated haploid eggs by the system of meiotic hybridogenesis (Morishima et al. 2008). In bisexually reproducing tetraploid loach with 4n = 100, most meiotic configurations comprised a large number of bivalents and several quadrivalents, suggesting an immediate re-diploidizing process of autotetraploids (Li et al. 2011). Such differences in reproductive modes may be closely linked to the genomic composition of unisexual diploids and polyploids that occurred in the process of polyploidization and hybridization of Misgurnus loaches (Arai and Fujimoto 2013).
In the present polyploids, quadrivalents, trivalents and univalent of Cobitis were not observed in GVs, and only bivalents were recognized. These situations may be related to their genomic composition, which allows the stable formation of unreduced eggs. In the system of premeiotic endomitosis, each homologous chromosome could not find any counterpart chromosome to pair before meiosis, and thus each homologous chromosome should be duplicated before entering into meiosis then to form bivalents between two sister chromosomes from the same chromosome (Arai and Fujimoto 2013). Thus, duplicated sister chromosomes behave as homologous chromosomes to form bivalents in the quasi-normal meiotic process and proceeded to the meiotic process to produce unreduced eggs. In this system, crossing over happens in each sister chromosome pair, but genetic variation never occurs because of the exchange of the same elements.
Considering previously obtained results, tetraploid females of Cobitis may be derived from the fertilization of triploid eggs with the sperm of Cobitis taenia males as well as gynogenetic reproduction of tetraploid females. The present results showed that tetraploid females generated tetraploid eggs which presumably develop gynogenetically. It is noteworthy that tetraploid males originated only as a result of fertilization of triploid eggs, but they are sterile because their testes contain no spermatozoa (Juchno and Boroń 2006). Very low frequencies (2–3 %) of tetraploid females and males of Cobitis in the Bug River population demonstrate that the formation of tetraploids is characterized by a very small efficiency. Furthermore, it is also affected by a smaller number of eggs produced by tetraploid females, than those of diploids and triploids (Juchno et al. 2007), and higher mortality of tetraploid larvae (Juchno et al. 2014).
References
Arai K, Fujimoto T (2013) Genomic constitution and atypical reproduction in polyploid and unisexual lineages of the Misgurnus loach, a teleost fish. Cytogenet Genome Res 140: 226–240
Boroń A (2003) Karyotypes and cytogenetic diversity of the genus Cobitis (Pisces, Cobitidae) in Poland: a review. Cytogenetic evidence for a hybrid origin of some Cobitis triploids. Folia Biol 51 (Suppl):49–54
Comai L (2005) The advantages and disadvantages of being polyploid. Nature Rev Genet 6:836–846
Gregory TR, Mable BK (2005) Polyploidy in animals. In: Gregory, TR (ed) The evolution of the genome. Elsevier Academic Press, London, pp 428–517
Itono M, Morishima K, Fujimoto T, Bando E, Yamaha E, Arai K (2006) Premeiotic endomitosis produces diploid eggs in the natural clone loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J Exp Zool 305A:513–523
Janko K, Flajšhans M, Choleva L, Bohlen J, Šlechtová V, Rábová M, Lajbner Z, Šlechta V, Ivanova P, Dobrovolov I, Culling M, Persat H, Kotusz J, Ráb P (2007) Diversity of European spined loaches (genus Cobitis L.): an update of the geographic distribution of the Cobitis taenia hybrid complex with a description of new molecular tools for species and hybrid determination. J Fish Biol 71 (Suppl C):387–408
Janko K, Kotusz J, De Gelas K, Šlechtová V, Opoldusová Z, Drozd P, Choleva L, Popiołek M, Baláž M (2012) Dynamic formation of asexual diploid and polyploid lineages: multilocus analysis of Cobitis reveals the mechanisms maintaining the diversity of clones. PLoS ONE 7:e45384
Juchno D, Boroń A (2006) Comparative histology of the testes of the spined loach Cobitis taenia L. and natural allotetraploids of Cobitis (Pisces, Cobitidae). Hydrobiologia 573:45–53
Juchno D, Boroń A, Gołaszewski J (2007) Comparative morphology and histology of the ovaries of the spined loach Cobitis taenia L. and natural allopolyploids of Cobitis (Cobitidae). J Fish Biol 70:1392–1411
Juchno D, Jabłońska O, Boroń A, Kujawa R, Leska A, Grabowska A, Nynca A, Świgońska S, Król M, Spóz A, Laskowska N, Lao M (2014) Ploidy-dependent survival of progeny arising from crosses between natural allotriploid Cobitis females and diploid C. taenia males (Pisces, Cobitidae). Genetica 142:351–359
Kottelat M (2012) Conspectus cobitidum: an inventory of the loaches of the world (Teleostei: Cypriniformes: Cobitoidei). Raffles Bull Zool 26 (Suppl):1–199
Lees J, Saat T (2003) Oocyte final maturation in tetraploid spined loaches (Cobitis) from the Moscow river. Folia Biol 51:75–78
Li YJ, Yu Z, Zhang MZ, Qian C, Abe S, Arai K (2011) The origin of natural tetraploid loach Misgurnus anguillicaudatus (Teleostei: Cobitidae) inferred from meiotic chromosome configurations. Genetica 139:805–811
Li YJ, Gao YC, Zhou H, Ma HY, Li JQ, Arai K (2015) Meiotic chromosome configurations in triploid progeny from reciprocal crosses between wild-type diploid and natural tetraploid loach Misgurnus anguillicaudatus in China. Genetica 143:555–562
Morishima K, Yoshikawa H, Arai K (2008) Meiotic hybridogenesis in triploid Misgurnus loach derived from a clonal lineage. Heredity 100:581–586
Nabais C, Pereira C, Cuńado N, Collares-Pereira MJ (2012) Synaptonemal complexes in the hybridogenetic Squalius alburnoides fish complex: new insights on the gametogenesis of allopolypoids. Cytogenet Genome Res 138:31–35
Otto SP (2007) The evolutionary consequences of polyploidy. Cell 131:452–462
Saitoh K, Kim I-S, Lee E-H (2004) Mitochondrial gene introgression between spined loaches via hybridogenesis. Zool Sci 21:795–798
Saitoh K, Chen WJ, Mayden RL (2010) Extensive hybridization and tetrapolyploidy in spined loach fish. Mol Phylogenet Evol 56:1001–1010
Yamashita M, Jiang J, Onozato H, Nakanishi T, Nagahama Y (1993) A tripolar spindle formed at meiosis I assures the retention of the original ploidy in the gynogenetic triploid Crucian Carp, Ginbuna Carassius auratus langsdorfii. Dev Growth Differ 35:631–636
Zhang Q, Arai K, Yamashita M (1998) Cytogenetic mechanisms for triploid and haploid eggs formation in triploid loach Misgurnus anguillicaudatus (Pieces: Cobitidae). J Exp Zool 281:608–619
Acknowledgments
We would like to express our sincere thanks to the Japan Society for the Promotion of Science (JSPS) for the Invitation Fellowship Program for Research (A. Boroń, ID No. S-05135) at the Faculty of Fisheries Sciences, Hokkaido University, in the 2005 fiscal year and Poland–Japan Research Cooperative Program in the 2015 to 2016 fiscal years. The authors also thank three Reviewers for helpful comments and suggestions for improving the manuscript. This work was also supported in part by grant N N303 068834 of the Polish National Science Center (NCN). All experiments comply with the current laws of Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Juchno, D., Arai, K., Boroń, A. et al. Meiotic chromosome configurations in oocytes of Cobitis taenia and its polyploid hybrids. Ichthyol Res 64, 240–243 (2017). https://doi.org/10.1007/s10228-016-0556-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0556-1