Abstract
The distribution of the Kildin cod Gadus morhua kildinensis in Lake Mogilnoye (Kildin Island, Barents Sea) is assessed based on the results of echo sounding and hydrological surveys, underwater photography and field observations. Underyearlings live in the lower part of the freshened upper layer, younger age groups locate in vicinity of the natural dam in the southeastern part of the lake, adults are distributed along the water area of the lake within the depth range of 4.0–6.4 m (mainly 5.0–5.4 m), characterized by favorable temperature values, water salinity, and oxygen saturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Kildin cod Gadus morhua kildinensis inhabits the subpolar meromictic relict marine Lake Mogilnoe located on Kildin Island (Barents Sea) (Svetovidov, 1948; Nikol’skii, 1971; Titov et al., 2002); the lake was formed about 1500 years ago by separating of the sea bay from the water area of Kildin Salma Strait as a result of the complex impact of a number of factors: seabed uplift, glacial accumulation, abrasion-accumulative activity of the sea, etc. (Deryugin, 1925; Gurevich and Liiva, 1975; Kotsubko and Kravchenko, 2002; Mityaev et al., 2008). Due to the small size of the lake, the population size of Kildin cod is limited; therefore, this subspecies is listed in the Red Lists of Russia and of the Murmansk Oblast (Shilin, 2001; Andreev et al., 2015; Stroganov et al., 2015; Zhivotovsky et al., 2016).
For more than two centuries, the cod population inhabiting Lake Mogilnoe has attracted the researchers’ attention. Fish morphology, biology, parasite fauna, nutrition, habitat conditions and their variability were studied (Ozeretskovskii, 1804; Fausek, 1891; Deryugin, 1925; Esipov, 1930; Dogel, 1936; Tseeb, 1975; Tseeb and Astafieva, 1975; Antsiferov and Trofimov, 2002; Karasev, 2002; Mukhina et al., 2002; Novikov et al., 2006). In our previous studies, the genetic parameters of Kildin cod were considered (Novikov et al., 2006; Stroganov et al., 2011), the origins of its extremely high genetic differentiation from the maternal population of the Atlantic cod G. morhua of the Norwegian-Barents Sea region and the mechanisms of adaptation to specific conditions of Lake Mogilnoe have been suggested (Stroganov et al., 2015, 2017).
The research aims to study the distribution and other biological characteristics of Kildin cod at different ontogenetic stages in regard to abiotic environmental conditions using echo sounding and underwater photography.
MATERIALS AND METHODS
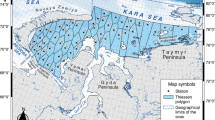
The material was collected during complex expeditions of Lomonosov Moscow State University, St. Petersburg State University, and PINRO to Lake Mogilnoe (Kildin Island, Barents Sea) in July–August of 2011, 2012, 2017, 2018, and 2020. The scheme of echo sounding and hydrological surveys is presented at Fig. 1. Previously published morphobiological and genetic data were used as well (Novikov et al., 2006; Stroganov et al., 2011, 2013).
Scheme of surveys on Lake Mogilnoe: (─)—transects nos. 1–12 of the echo sounding survey on July 25–30, 2012, (––)—transects nos. I–IV of the echo sounding survey on August 3, 2017, (◇)—echo sounding survey along the natural dam on July 30, 2017; (◻)—stations nos. 1–5 of hydrological survey on July 28, 2017, (◼)—hydrological survey station on August 12–13, 2020; (▼)—location of the trap camera on July 26, 2018. Scale bar: 50 m.
The fish were caught with hook gear with alternate fishing in different parts of the lake according to the catch-and-release principle (in accordance with the permits of the Russian Ministry of Natural Resources and Ecology). The movement of fishing gear was forced when cod was not caught in the fished place for a day or more (despite the fact that the caught individuals were almost immediately released into the lake). Caught fish (75 specimens, 2011–2012) were photographed, their total length (TL) and body weight were measured.
Echo sounding surveys were carried out to study the features of the distribution and behavior of Kildin cod using a Humminbird PiranhaMAX 230e portable echo sounder (Fig. 1). In 2012, 12 transects in triplicates were performed perpendicular to the longitudinal axis of the lake, distributed relatively evenly over its water area. In 2017, in addition to four transects perpendicular to the longitudinal axis of the lake, echo sounding was performed along the natural dam with the direction of the echo sounder beam perpendicular to the underwater part of its slope to study the distribution of cod in the water area adjacent to the natural dam (in triplicates).
A trap camera (a BRINNO BPC100 video camera with interval shooting in a sealed transparent box) was used to obtain underwater photographs of cod; the camera was installed by a SCUBA diver at a 4-m depth nearby a natural dam in the southeastern part of the lake (Fig. 1).
The bathometric method of sampling was used (Shevlyakova, 2017) using an Alekseev system bathometer for conducting hydrological surveys (Fig. 1). In 2017, water temperature was measured with a mercury thermometer with a division value of 0.5°C, water salinity, with a Kelilong RHS-28BATC salinity refractometer with a division value of 1‰. The measurements were carried out from the surface down to the bottom at five stations, distributed relatively evenly along the median longitudinal transect of the lake surface. In 2020, water temperature, water salinity, and the dissolved oxygen content were recorded at different depth in the central zone of the southeastern part of the lake with a S/Mill-E refractometer (Atago) and Expert 009 submersible thermometer-oximeter (Econix-Expert Ltd).
RESULTS
During the echo sounding survey on July 25–30, 2012, 278 cod specimens (adults and large recruits) were registered on average (triplicates) in Lake Mogilnoe. In the northwestern part of the lake, they were distributed fairly evenly (along the transects, one sixth of individuals ranged from 10 to 12%), along other transects, the proportion of individuals decreased in the southeast direction (from 7 to 2%) (Fig. 2).
Relative abundance of Kildin cod Gadus morhua kildinensis along 12 transects of echo sounding survey (July 25–30, 2012). The average values (triplications) relative to the average number of registered large individuals and recruits (278 specimens) are given; the numbering of transects are similar to Fig. 1.
The main purpose of the survey, carried out on August 3, 2017, was to determine the bathymetric distribution of Kildin cod in the lake at four transects along the longitudinal axis of the lake. According to echo sounding data, all recorded individuals (114 specimens) occupied the depth range of 4.0–6.4 m; along all transects, most individuals (from 56 to 78, 68.6% on average) occupied the 5.0–5.4-m water layer (Fig. 3).
An echo sounding survey (July 30, 2017) along the natural dam, separating the lake from Kildin Salma Strait, revealed here the cod presence (28 specimens). The echo sounder model used for our study made it possible to distinguish between the sizes of fish only as large and small. It should be noted that in 2011 and 2012 large specimens (TL > 32.5 cm and weight > 400 g) were noted in the catches throughout the entire water area of the lake. An exception was the water area along the natural dam, where specimens of TL 13–14 cm weighing ~15 g, belonging to the age group of two-year-olds, were very rarely caught along with large cod (Chugunova, 1959; Mukhina et al., 2002). We assumed that this size group was identified by the echo sounder as small. According to the survey data, their share averaged 51% of the total number of individuals recorded in the dam zone, they located at a 4–7-m distance from the dam. Cod specimens, determined by the echo sounder as large, were distributed at a greater distance from the natural dam, from 4 to 26 m.
Lake Mogilnoe is characterized by vertical water stratification, expressed both in terms of water temperature, water salinity, and oxygen content. According to the data of the hydrological survey performed on July 28, 2017 (Table 1), the upper margin of the thermocline corresponded to a 3-m depth, the lower one, to 8-m depth; the upper and lower margins of the halocline were 3–4 m and 7–8 m, respectively. The boundary between unstable highly desalinated waters and more saline deep waters was drawn along the 3-m isobath. It should be noted that significant desalination revealed at late July 2017 in the northwestern part of the lake, registered deeper than 8-m depth, had most likely a time-limited character and was associated with the formation of stratification along the longitudinal axis of the lake due to calm weather during several days against the background of specific correlation between fresh and sea water inflow zones in the Lake Mogilnoe (Gurevich and Liiva, 1975). A single similar situation was noted earlier (Strelkov et al., 2019).
In early August 2020, in the central zone of the southeastern part of the lake, the thermohaline characteristic was similar to that at the end of July 2017, but some peculiarities were observed. The temperature of the surface water layer (0–2 m) was somewhat lower than at a depth of 3–4 m (13.7 versus 15.0–16.0°C). In the depth range of 5–9 m, the temperature decreased sharply (from 13.2 to 9.1°C); deeper than 9 m and down to the bottom, it was stable (8.8–8.9°C). The salinity was 4‰ in the 0–2-m water layer; it sharply increased from 9 to 25‰ in the depth range of 3–7 m; then it gradually increased up to 29‰ below 7-m depth and down to the bottom. The oxygen content in the surface water layer (0–5 m) corresponded to 100% saturation and even slightly exceeded it (up to 115% at a 3-m depth); in the depth range of 5–7 m, this indicator decreased from 88 to 11%; deeper than 7 m and down to the bottom, it was extremely low (8–10%), unsuitable for the normal life of fish.
In 2018, underwater photographs of Kildin cod were obtained for the first time using a trap camera (Fig. 4). Based on the size of the sea anemones at the photo, we assumed that this was a large sexually mature individual (~60-cm long). According to the image sharpness, the position of the chin barbel, ventral fins and caudal fin, this cod floated in the water column in one place or slowly moved due to the undulating of the pectoral fins. The conclusion about the territorial way of life of Kildin cod (Stroganov et al., 2017) was confirmed by the shore observations of large individuals that remained in the same places for several days (Appendix 1).
In addition to large cod, underwater photographs of juveniles were obtained for the first time. The block of photographs given in Appendix 2 demonstrates the movement of flocks of juvenile cod in the pelagial, as well as their approaches to the coastal zone in the area of the natural dam slope. Considering the size of individuals at the photographs (approximately 5–6 cm) and their distribution in the water column, flocks were formed by offspring from the spawning of the current year, attributed to the young-of-the-year group, including by size parameters (Chugunova, 1959; Mukhina et al., 2002). Such flocks were visually observed throughout the entire water area of the lake, including at shallow depths (~1 m).
Fundamental differences were revealed when comparing the coloration of underyearlings, two-year-olds, and larger individuals of Kildin cod. The bright spotted coloration of large individuals corresponds to that described in the literature and even used for taxonomic identification of Kildin cod (Svetovidov, 1948). Medium-sized bright spots (2–3 mm in diameter), brown with a fulvous tint, framed by a light border, densely cover the back and upper part of the lateral surface of the fish body, extending over the head and dorsal fins. The lower jaw, throat and belly are bright white. According to our data, this coloration is characteristic of fish of a wide age range, from 3 to 12 years. The coloration of underyearlings and two-year-olds of Kildin cod is radically different: it is faded, without bright spots. The back is brownish-olive, large weakly expressed yellowish-brown spots of irregular shape are visible on the sides above and slightly below the lateral line on a light background. The lower jaw, throat, and belly of these fish are yellowish-white.
DISCUSSION
Comparison of the results of echo sounding surveys in 2012 and 2017 and the data of rod fishing allows us to assume a fairly stable state of the cod group in the lake. According to the echo sounding survey data, there are differences in the cod distribution along the transects in the northwestern and southeastern parts of the lake; however, this phenomenon is apparently related to a greater extent with the morphometric characteristics of the lake (Gurevich, 1975). Earlier, we have found that Kildin cod modifies its feeding behavior in order to ensure survival in the conditions of Lake Mogilnoe by mastering the feeding strategy and even by switching its lifestyle similar to the territorial species (Stroganov et al., 2015, 2017). Individuals of Kildin cod stay within a certain area as indicated by the observations presented in this paper. Firstly, cod individuals stay at same places and depth when recording by an echo sounder during entire survey, they do not react to a boat moving along the surface; secondly, there are visual observations of individuals remaining at certain place for days (we provide a photo of cod that stayed at the same place and depth for a week near the expedition camp in 2015). Such a relatively sedentary, territorial way of life, which differs from the Atlantic cod individuals constantly moving in search of food in the offshore and coastal waters of the Barents and Norwegian Seas (Boitsov et al., 2003; Orlova, Dolgov, 2004), is due not only to the small size of Lake Mogilnoe, but contributes to energy economy in the conditions of shortage of food suitable for large sexually mature individuals of Kildin cod (Brett, 1983). In this situation, the term “stationary” may be applied to Kildin cod, which is assigned by some authors to groups of Atlantic cod with lower migratory activity (Wentzel-Larsen and Nordeide, 2001; Nordeide et al., 2011; Teterina and Zhivotovskii, 2017).
Large cod is practically not found in Lake Mogilnoe outside the depth range of 4.0–6.4 m; moreover, most of individuals (68.6%) occupy narrow depth range of 5.0–5.4 m. Kildin cod is a subspecies of the Atlantic cod, it is a representative of the boreal ichthyofauna, so this species retains the corresponding preferences in the ranges of abiotic factors (in particular, water temperature and salinity). In Lake Mogilnoe, the depth range of 5.0–5.4 m is apparently characterized by environmental parameters that are comfortable for adult cod. In particular, here the water temperature in summer decreases down to 9–11°C, unlike overheated surface layers (Antsiferov and Trofimov, 2002; Krasnova et al., 2019; present study). The water salinity in the subsurface layer of the lake varies within 2–5‰ due to freshening due to precipitation and inflow of groundwater. According to experimental data, Kildin cod is able to stay in the water mass with such low salinity for quite a long time without obvious malfunctioning (Tseeb, 1975). Water salinity in the lake increases rapidly as the depth increases and reaches 20–25‰ at a depth of 5.0–5.5 m (Antsiferov and Trofimov, 2002; Krasnova et al., 2019; present study). When considering oxygen saturation of water in Lake Mogilnoe, the upper desalinated zone is the richest by this parameter, where it reaches 100% and even higher due to the photosynthetic activity of phytoplankton. However, the water layer with a high oxygen content extends down to a 5-m depth, this indicator decreases sharply below this depth, reaching as less as ~50% at a 6-m depth, as shown by our results and the data of other studies performed in 2010s (Strelkov et al., 2014, 2019; Krasnova et al., 2019).
Therefore, the distribution of cod in Lake Mogilnoe within a narrow depth range (5.0–5.4 m) is governed by the abiotic factors: the upper boundary depends on the water temperature and salinity, the lower one, on the oxygen content. This does not exclude short migrations of some individuals outside the upper boundary of the habitat zone (for example, for feeding). Earlier, large cod specimens overturning flat rounded stones and feeding on amphipods in the coastal zone of the lake near the water’s edge have been described (Serebrov et al., 2002).
The younger age groups of cod are difficult to study in Lake Mogilnoe. This applies to a greater extent to the juveniles at the age of 1+ and 2+, which lead a secretive lifestyle, including because of the risk of being food objects for large Kildin cod individuals (Mukhina et al., 2002; Serebrov and Ignashkin, 2002). We have observed the cases of cannibalism in 2011 and 2012, when a small individual caught on a hook was captured by a large cod. According to the catches and echo sounding data, cod juveniles have been recorded only in the dam zone in the southeastern part of Lake Mogilnoe. In our opinion, this is due to the peculiarities of the topography of the underwater slope of the natural dam, where rounded boulders (0.25–1.00 m in diameter) and large stones and rocky ground, sometimes overgrown with macrophytes, provide juveniles with a large number of shelters to escape large cod, which increases their survival rate. It should be noted that the other shores of the lake are sloping, with a certain degree of silting; there are no reliable shelters for juveniles. In addition, significant seeping of salty waters of the Barents Sea into the lake in the dam zone contributes apparently to an increase both of fodder base and of the growth rate of cod juveniles (Tseeb, 1975; Mukhina et al., 2002).
For the first time, the images obtained by a trap camera provide the information on the peculiarities of the ecology of Kildin cod underyearlings before they start a demersal lifestyle. It has been found that underyearlings move in flocks in the lower part of the freshened upper layer, they also approach the coastal zone in the area of the natural dam slope. The yellowish-brown coloration of underyearlings with large spots completely coincides with the coloration of two-year-olds and radically differs from the bright coloration of large Kildin cod. It should also be noted that the coloration of the underyearlings of Kildin cod corresponds to that of early juveniles of the Atlantic cod and Pacific cod G. macrocephalus (Rass, 1946; Auditore et al., 1994; Voskoboinikova et al., 2012) and completely differs from the description given earlier (Tseeb, 1975).
The results of our complex studies, along with the literature data, allow us to get an idea of the features of the biology of Kildin cod in different periods of life as a fairly complete picture. The development of pelagic eggs takes place in the lower marine layer (depth range of 6.5–7.5 m, water salinity 26–27‰); pre-larvae and feeding larvae also locate here (Tseeb and Pozdnyakov, 1975). After metamorphosis, transition to the juvenile state and development of hydrostatic, motor and other functions, underyearlings get the opportunity to expand their habitat area: they master the desalinated water layer. At the beginning of the autumn period, juveniles leave the pelagial and descend to the bottom to lead demersal lifestyle, preferring the area along the natural dam (southeastern part of the lake) and the water area adjacent to it. Juveniles of Kildin cod seem to spend one to two years under the protection of coarse-grained material of the dam. Such location of juveniles protects them from excessive predation by large cod and thus ensures the stability of recruitment of the Kildin cod spawning group in the lake. Older fish are distributed fairly evenly over the water area of the lake, preferring saline water layer (depths of 5.0–5.4 m).
As a corollary, it should be noted that studies carried out in Lake Mogilnoe over time indicated relative stability of population of Kildin cod (Titov, 2002; Stroganov et al., 2015). However, additional studies are necessary to assess the exact size of this population. Currently, according to the IUCN Red List criteria (IUCN, 2012), Kildin cod belongs to the category of the most threatened species (critically endangered, CR); similar category (critically endangered, CR) is assigned to this species in the Red List of Russian Federation (https://docs.cntd.ru/document/564578614). This is due to its small range and gradually narrowing living space due to the growth of the hydrogen sulfide layer. This requires constant monitoring of both the state of the lake ecosystem and the response of Kildin cod population to these changes, which are the result of a unique nature experiment (Titov, 2002; Zhivotovsky et al., 2016).
REFERENCES
Andreev, V., Fokin, M., Mugue, N., and Strelkov, P., Long-term persistence and evolutionary divergence of a marine fish population with a very small effective population size (Kildin cod Gadus morhua kildinensis), Mar. Biol., 2015, vol. 162, pp. 979–992. https://doi.org/10.1007/s00227-015-2642-8
Andriyashev, A.P., Ryby severnykh morey SSSR (Fishes of the Northern Seas of the USSR), Moscow: Akad. Nauk SSSR, 1954.
Antsiferov, M.Yu. and Trofimov, A.G., Hydrological conditions, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, pp. 20–33.
Auditore, P.J., Lough, R.G., and Broughton, E.A., A review of the comparative development of Atlantic cod (Gadus morhua L.) and haddock (Melanogrammus aeglefinus L.) based on an illustrated series of larvae and juveniles from Georges Bank, NAFO Sci. Counc. Stud., 1994, no. 20, pp. 7–18.
Boitsov, V.D., Lebed’, N.I., Ponomarenko, V.P., et al., Treska Barentseva morya: biologiya i promysel (The Cod of the Barents Sea: Biology and Fishery), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2003.
Brett, J.R., Environmental factors and growth, in Fish Physiology, Vol. 8: Bioenergetics and Growth, Hoar, W.S., Randall, D.J. and Brett, J.R., Eds., Amsterdam: Elsevier, 1979, ch. 10.
Chugunova, N.I., Rukovodstvo po izucheniyu vozrasta i rosta ryb (Manual for Study of Age and Growth of Fishes), Moscow: Akad. Nauk SSSR, 1959.
Deryugin, K.M., Relict Lake Mogilnoye, Tr. Petergof. Estestv.-Nauchn. Inst., 1925, no. 2, pp. 1–98.
Dogel’, V.A., Parasites of cod from the Lake Mogilnoye, Uch. Zap. Leningr. Gos. Univ., Ser. Biol., 1936, no. 3, no. 7, pp. 123–133.
Esipov, V.K., The cod from the Lake Mogilnoye on the Kildin Island in the Barents Sea, Gidrobiol. Zh., 1930, vol. 9, nos. 4–6, pp. 131–137.
Fausek, V.A., In the far north: from a trip to the White Sea and the ocean, Vestn. Evropy, 1891, no. 8, pp. 665–714.
Gurevich, V.I., Relief, bathymetry, and morphometry, in Reliktovoe ozero Mogil’noe (Relict Lake Mogilnoye), Leningrad: Nauka, 1975, pp. 18–21.
Gurevich, V.I. and Liiva, A.A., Age of the Lake Mogilnoye, in Reliktovoe ozero Mogil’noe (Relict Lake Mogilnoye), Leningrad: Nauka, 1975, pp. 102–104.
IUCN, Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: Version 4.0, Gland: IUCN, 2012.
Karasev, A.B., Parasitic fauna of fishes, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, pp. 81–87.
Kotsubko, E.V. and Kravchenko, A.S., Characteristic of the watershed squire, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, p. 19.
Krasnova, E.D., Efimov, V.A., Fedyuk, M.L., et al., New data on Lake Mogilnoe (Kildin Island, Barents Sea): the results of the 2018 expeditions, IOP Conf. Ser.: Earth Environ. Sci., 2019, vol. 263, pp. 1–8. https://doi.org/10.1088/1755-1315/263/1/012019
Mityaev, M.V., Korsun, S.A., Strelkov, P.P., and Matishov, G.G., Ancient coastlines of Eastern Kildin, Dokl. Earth Sci., 2008, vol. 423, no. 2, pp. 1455–1458.
Mukhina, N.V., Lepesevich, N.A., and Filina, E.A., Biological state of the Kildin cod, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, pp. 88–109.
Nikol’skii, G.V., Chastnaya ikhtiologiya (Particular Ichthyology), Moscow: Vysshaya Shkola, 1971.
Nordeide, J.T., Johansen, S.D., Jorgensen, T.E., et al., Population connectivity among migratory and stationary cod Gadus morhua in the Northeast Atlantic—a review of 80 years of study, Mar. Ecol.: Progr. Ser., 2011, vol. 435, pp. 269–283. https://doi.org/10.3354/meps09232
Novikov, G.G., Stroganov, A.N., and Vinogradskii, V.S., Morphobiological caracteristics of Kildin cod, Tr. Belomorsk. Biol. Stn. Mosk. Gos. Univ., 2006, vol. 10, pp. 110–116.
Orlova, E.L. and Dolgov, A.V., Long-term nutritional strategy of cod in an unstable food supply, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2004, vol. 137, pp. 85–100.
Ozeretskovskii, N.Ya., Opisanie Koly i Astrakhani (Description of Kola Region and Astrakhan), St. Petersburg: Imper. Akad. Nauk, 1804.
Rass, T.S., Stages of ontogenesis of Teleostei fishes, Zool. Zh., 1946, vol. 25, no. 2, pp. 137–148.
Serebrov, L.I. and Ignashkin, V.A., Assessment of population size of the Kildin cod, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, pp. 110–116.
Serebrov, L.I., Drobysheva, S.S., Rocheva, E.V., and Dolgov, A.V., Abundance and biomass of benthic amphipods in the coastal area, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, pp. 77–81.
Shevlyakova, A.D., Bathometer: concept, use, and purpose, Byull. Med., 2017, vol. 7, no. 1, pp. 312–313.
Shilin, N.I., The Kildin cod Gadus morhua kildinensis Derugin, 1920, in Krasnaya kniga Rossiiskoi Federatsii. Zhivotnye (The Red Data Book of the Russian Federation. Animals), Moscow: AST Astrel’, 2001, pp. 308.
Strelkov, P., Shunatova, N., Fokin, M., et al., Marine Lake Mogilnoe (Kildin Island, the Barents Sea): one hundred years of solitude, Polar Biol., 2014, vol. 37, pp. 297–310. https://doi.org/10.1007/s00300-013-1431-4
Strelkov, P., Stogov, I., Krasnova, E., et al., Rapid unpredicted changes in the stratification of marine Lake Mogilnoe (Kildin Island, the Barents Sea) through the early 21st century, Polar Res., 2019, vol. 38, pp. 1–7. https://doi.org/10.33265/polar.v38.3394
Stroganov, A.N., Afanasiev, K.I., Rubtsova, G.A., et al., Data on variation of microsatellite loci in Kildin cod Gadus morhua kildinensis (Gadidae), J. Ichthyol., 2011, vol. 51, no. 7, pp. 500–507. https://doi.org/10.1134/S0032945211040187
Stroganov, A.N., Mukhina, N.V., Afanas’ev, K.I., et al., Complex expeditions to the Lake Mogilnoye (Kildin Island, Barents Sea) in 2011 and 2012, Vestn. Astrakhan. Gos. Tekh. Univ., Ser. Rybn. Khoz., 2013, no. 3, pp. 86–90.
Stroganov, A.N., Kriksunov, E.A., Zuykova, N.V., et al., The biological features of the Kildin cod, Gadus morhua kildinensis Derjugin, 1920 (Gadidae), Russ. J. Mar. Biol., 2015, vol. 41, no. 6, pp. 424–431. https://doi.org/10.1134/S1063074015060140
Stroganov, A.N., Afanasiev, K.I., Burmensky, V.A., et al., Mechanisms of the adaptation of the Kildin cod Gadus morhua kildinensis Derjugin, 1920 (Pisces: Gadidae) to the specific conditions of Lake Mogilnoye, Russ. J. Mar. Biol., 2017, vol. 43, no. 2, pp. 132–139. https://doi.org/10.1134/S1063074017020122
Svetovidov, A.N, Fauna SSSR. Ryby. Tom 9. Vyp. 4. Treskoobraznye (Fauna of the Soviet Union: Fishes, Vol. 9, No. 4: Gadiformes Fishes), Moscow: Akad. Nauk SSSR, 1948.
Teterina, A.A. and Zhivotovsky, L.A., DNA markers for identification of stationary and migratory ecotypes of Atlantic cod Gadus morhua, Russ. J. Genet., 2017, vol. 53, no. 7, pp. 834–837. https://doi.org/10.1134/S1022795417070122
Titov, O.V., Conclusions, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, pp. 141–156.
Titov, O.V., Serebrov, L.I., and Karasev, A.B., Introduction, in Reliktovoe ozero Mogil’noe (issledovaniya 1997–2000 gg.) (Relict Lake Mogilnoye: Studies of 1997–2000), Murmansk: Polar. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2002, pp. 3–17.
Tseeb, R.Ya., Gadus morhua kildinensis Derjugin, in Reliktovoe ozero Mogil’noe (Relict Lake Mogilnoye), Leningrad: Nauka, 1975, pp. 220–222.
Tseeb, R.Ya. and Astaf’eva, A.V., Morphology of the Kildin cod, in Reliktovoe ozero Mogil’noe (Relict Lake Mogilnoye), Leningrad: Nauka, 1975, pp. 259–276.
Tseeb, R.Ya. and Pozdnyakov, Yu.F., Reproduction, in Reliktovoe ozero Mogil’noe (Relict Lake Mogilnoye), Leningrad: Nauka, 1975, pp. 227–247.
Voskoboinikova, O.S., Nazarkin, M.V., and Golubova, E.Yu., Earlier stages of fishes from the northern part of the Sea of Okhotsk, in Issledovaniya fauny morei (Explorations of Fauna of the Seas), St. Petersburg: Zool. Inst., Ross. Akad. Nauk, 2012, vol. 68 (76), pp. 1–108.
Wentzel-Larsen, T. and Nordeide, J.T., Testing for homogeneity in sampling from sympatric noninterbreeding populations based on gene frequencies: the case of stationary and migratory cod, Sarsia, 2001, vol. 86, pp. 229–235. https://doi.org/10.1080/00364827.2001.10420479
Zhivotovsky, L.A., Teterina, A.A., Mukhina, N.V., et al., Effects of genetic drift in a small population of Atlantic cod (Gadus morhua kildinensis Derjugin) landlocked in a meromictic lake: genetic variation and conservation measures, Conserv. Genet., 2016, vol. 17, pp. 229–238. https://doi.org/10.1007/s10592-015-0774-5
SUPPLEMENTARY INFORMATION
Additional information for this article is available at doi 10.31857/S0042875222030225 for authorized users.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Martynova
Supplementary Information
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stroganov, A.N., Strelkov, P.P., Shilin, N.I. et al. New Data on the Biology of Kildin Cod Gadus morhua kildinensis (Gadidae) from Lake Mogilnoe (Kildin Island, the Barents Sea) Obtained by Echo Sounding and Underwater Photography. J. Ichthyol. 62, 586–593 (2022). https://doi.org/10.1134/S003294522203016X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003294522203016X