Abstract
Experimental data were summarized to assume that dinitrosyl iron complexes (DNICs) with thiol-containing ligands are an endogenous “working form” of the nitric oxide (NO) system in living organisms. DNICs can function as donors of both neutral NO molecules, which are responsible for positive regulatory effects of the NO system on various physiological and biochemical processes in humans and animals, and nitrosonium cations (NO+), which are responsible mostly for negative cytotoxic activity of the system. Special attention is paid to the finding that DNICs, especially in combination with dithiocarbamate derivatives, suppress SARS-CoV-2 infection in Syrian hamsters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As has been established to date, the simplest chemical compound nitric oxide (nitrogen monoxide, NO) is produced enzymatically in cells of all living organisms, including humans, animals, plants, and microbes, and functions both as a positive and as a negative regulator of almost all metabolic processes [1–3]. Activation of metabolic processes is a positive effect of NO, while its negative effect consists in their suppression and cytotoxicity.
It is clear that the life of NO as an active free-radical molecule in a free (unbound) state cannot be long enough for its paracrine and autocrine effects in living organisms. To ensure the effects, NO is naturally incorporated in endogenous compounds, such as S-nitrosothiols (RS-NO) and dinitrosyl iron complexes (DNICs) with low- or high-molecular-weight (protein) ligands. The compounds transfer NO over substantial distances to the targets of its biological effects and subsequently release NO to allow its interactions with the targets [4–9].
As was reported by my team, RS-NO and DNICs are interconvertible and, therefore, interdependent in the body. DNICs can be converted to RS-NO, and RS-NO are converted again to DNICs in the presence of divalent iron. DNICs are thought to play a main role in the DNIC–RS-NO system because they are a component that determines the existence of the system [6, 8].
Recent studies showed that DNICs with thiol-containing ligands are capable of donating not only neutral NO molecules, but also nitrosonium cations (NO+) in the body [10–12]. This property is possibly responsible for DNIC cytotoxicity. Nitrosonium cations arise in DNICs in the mechanism of their formation, in which disproportionation of NO molecules binding with a divalent iron ion in a pairwise manner is the most important step [13]. The NO molecules are converted to a nitroxyl anion (NO–) and a nitrosonium cation as a result of the disproportionation reaction (Scheme 1). Nitroxyl anions bind protons to yield neutral nitroxyl molecules (HNO), which leave the ligand surrounding of iron. Another, third NO molecule occupies the resulting space to form a mononuclear DNIC (M-DNIC) with thiol-containing ligands. M-DNIC has the resonance structure [(RS–)2Fe2+(NO)(NO+)] according to Scheme 1. The nitrosonium cation might interact with the hydroxyl anion to yield nitrous acid, which would leave the complex. However, this does not take place and the nitrosonium cation remains in the complex because the electron density is transferred from the serum atoms of the thiol-containing ligands, which are nucleophilic DNIC components, to the nitrosonium cation, which is a potent electrophile. The transfer neutralizes the positive charge of the nitrosonium cation, thus preventing its interaction with the hydroxyl anion and ensuring DNIC preservation.

Scheme 1. Mechanism whereby mononuclear DNIC forms in the reaction of NO with Fe2+ and thiol-containing compounds (RS–) [12].
The disproportionation reaction between NO molecules, that is, their mutual one-electron reduction–oxidation, converts the initially diamagnetic iron-dinitrosyl moiety (IDNM) [Fe2+(NO)2] with an even number of electrons to a paramagnetic state with S = 1/2, which is characterized by a EPR signal with gmean = 2.03 (signal 2.03). Based on the signal, DNICs were detected and identified in living organisms as early as the 1960s [14–16].
When the concentration of thiol-containing compounds decreases, paramagnetic M-DNICs with thiol-containing ligands undergo reversible dimerization to produce diamagnetic binuclear DNICs (B-DNICs, resonance structure [(RS−)2Fe\(_{2}^{{2 + }}\)(NO)2(NO+)2]), which predominate over M-DNICs in animal tissues [17]. The diamagnetic character of B-DNICs is determined by spin pairing in two IDNMs via the sulfur bridges that connect the IDNMs in a B-DNIC. Their characteristic feature is an optical absorption spectrum (Fig. 1) with main bands at 310 and 360 nm with respective extinction coefficients ε of 4600 and 3700 M–1 cm–1 as related to one IDNM in B-DNIC [17]. The optical absorption spectrum of S-nitrosothiol (Fig. 1) has a main band at 334 nm (ε = 0.94 M–1 cm–1) and a weak band at 540 nm [18].
Both M- and B-DNICs with the IDNM resonance structure [Fe2+(NO)(NO+)] can similarly arise in the reaction of Fe2+, thiols, and S-nitrosothiols (RS‒-NO+), which bind in pairs with the Fe2+ ion and subsequently undergo disproportionation (Scheme 2), like NO molecules in Scheme 1.

Scheme 2. Mechanism whereby M-DNIC forms in the reaction of RS-NO with Fe2+ and thiol-containing compounds (RS–) [12].
The RS-NO molecules are converted to the unstable adducts (RS•-NO+) and (NO-RS–) as a result of disproportionation and are subsequently decomposed. A thiyl radical and ionized thiol (thiolate anion) are released from the complex, while NO and NO+ remain. This yields DNIC with the resonance structure [(RS–)2Fe2+(NO)(NO+) = (RS–)2Fe+-(NO+)(NO+)], the same as in the case where DNIC forms in the reaction of NO with Fe2+ and thiol-containing compounds (Scheme 1).
It is clear that a reaction shown in Scheme 3 describes chemical equilibrium between M-DNIC with a resonance structure shown in Schemes 1 and 2 and the components of the complex.
Scheme 3. Chemical equilibrium between M-DNIC and its components: thiols, Fe2+ ions, NO molecules, and nitrosonium cations [12].
According to Scheme 3, M-DNIC nitrosyl ligands can be released from the complex in the form of neutral NO molecules or in the form of nitrosonium cations, in equal proportions. The release is impossible if the resonance structure of DNICs with thiol-containing ligands is [(RS-)2Fe–1(NO+)2], which is assumed in many studies in the field. This resonance structure can be true indeed for DNICs in crystal, but does not characterize DNICs in solution in view of the arguments described previously [11, 12, 14]. Once chemical equilibrium is achieved for DNICs with the resonance structure [(RS–)2Fe–1(NO+)2], nitrosyl ligands can be released from the complexes only in the form of NO and NO–, but not in the form of nitrosonium cations [11, 12, 14].
It is commonly accepted now that DNICs with thiol-containing ligands act as NO stabilizers, transporters, and donors [7, 9]. As for the cytotoxic effect of DNICs, the majority of researchers who think that only NO molecules can be released from DNICs naturally attribute cytotoxic activity of DNICs to these NO molecules or, more exactly, peroxynitrite (ONOO–) resulting from their reaction with superoxide anions [7, 9]. Once protonated, peroxynitrite decomposes into a hydroxyl radical and nitrogen dioxide, which are potent cytotoxic agents [19, 20]. Nitrosonium cations were first assumed to act as efficient cytotoxic agents released from DNICs in a study of German and Russian researchers [21], who investigated the cytotoxic effect of M-DNIC with thiosulfate (M-DNIC-TS) in Jurkat tumor cell cultures. The effect was preserved in full when the complexes were destroyed using the dithiocarbamate derivative N‑methyl-D,L-glucamine dithiocarbamate (MGD).
Following the researchers, the result indicated unambiguously that a nitrosonium cation released from the complex is responsible for the cytotoxic effect of M-DNIC-TS on Jurkat cells. The conclusion stems from the mechanism whereby MGD as a dithiocarbamate derivative decomposes M- and B-DNICs with thiol-containing ligands (Scheme 4). DNICs are decomposed because MGD (dithiocarbamate) captures the iron-mononitrosyl group of IDNM to yield a mononitrosyl iron complex (MNIC) with MGD and to release a nitrosonium cation from M- and B‑DNICs.

Scheme 4 . Mechanism whereby dithiocarbamate decomposes M- and B-DNICs with thiol-containing ligands [22].
As a result, NO molecules are incorporated in stable MNICs with MGD and thereby drop out of the game, being no longer capable to affect cells and tissues. Nitrosonium cations are free and are therefore the only agent that can affect the biosystems.
Characteristically, the proapoptotic effect of M‑DNIC-TS increased in the presence of MGD [21]. M-DNIC-TS and MGD induce apoptosis when used alone, especially the former. If the two compounds did not affect each other when used together, they would induce apoptosis in 45% of cells as judged from published data [21]. However, more than 60% of cells actually became apoptotic when treated with both of the agents used together in a real experiment [21].
An increase in the M-DNIC-TS effect was mostly likely explained by decomposition of all complexes with the release of a maximum amount of nitrosonium cations, which caused apoptosis. It is of interest that glutathione decreased the proapoptotic effect of M‑DNIC-TS [21]. A lower effect was observed in the presence of glutathione probably because M-DNIC-TS formed a more stable complex with glutathione, B‑DNIC-GSH, which released nitrosonium cations in substantially lower amounts as compared with M‑DNIC-TS.
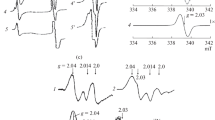
Diethyldithiocarbamate (DETC) is another dithiocarbamate derivative capable of decomposing M‑DNIC-TS according to Scheme 4. Taking advantage of this property, a hypotensive effect of M‑DNIC-TS as a NO donor was studied in animals (Fig. 2) [23]. DETC substantially reduced the effect when injected intravenously prior to M-DNIC-TS administration (Fig. 2, curve 3). When DETC was injected after M-DNIC-TS administration, a short-term abrupt drop in blood pressure (BP) was observed in the animals. BP dropped possibly because nitrosonium cations were reduced to NO within RS-NO, which formed as a result of DNIC decomposition (according to Scheme 4), and NO was released into the medium (Fig. 2; curves 4, 5). A weak effect that DETC exerted on BP that had already been lowered in these animals (curves 4, 5) was possibly explained by lack of contact between DETC and DNIC localized within cells [23].
Changes in blood pressure (BP and ∆BP) in awake rats after (1, 2) intravenous (i.v.) injection of M-DNIC-TC at 10 µmol/kg body weight; (3) M-DNIC-TS injection made 1 min after i.v. injection of DETC at 30 mg/kg; and (4, 5) M-DNIC-TS injection followed by DETC injection, which was made at time points indicated with arrows [23].
Likewise, MGD dramatically increased the cell death induced in MCF-7 cell cultures by incubation with B-DNIC with mercaptosuccinate (B-DNIC-MS) [22] A decrease in MCF-7 cell survival (or cell death) was assessed using a two-dimensional plot of flow cytometry data. The plot characterized the extents of apoptosis (inferred from annexin V-FITS fluorescence) and necrosis (inferred from propidium iodide fluorescence).
As is seen from Fig. 3, the dead cell portion reached 80% when B-DNIC-MS and MGD were added simultaneously to a cell culture (column 4), whereas the portion would not exceed 50% (column 5) if the agents acted separately without interacting with each other (columns 2, 3). It is clear that a synergistic effect was exerted by B-DNIC-MS and MGD added simultaneously to the cell culture and that the effect was due to a higher content of nitrosonium cations, which were released from B-DNIC-MS upon its interaction with MGD according to Scheme 4.
Death of MCF-7 human tumor cells (%). Columns: (1) контроль; (2) incubation with 0.5 mM B-DNIC-MS; (3) incubation with 1.0 mM MGD; (4) incubation with a mixture of the two agents; (5) sum of the effects exerted by B-DNIC-MS and MGD (columns 2 + 3) in the absence of their interaction [22].
A similar result was obtained for a combined effect of DETC and B-DNIC-GSH on the survival of Escherichia coli TN350 cells (cell viability was inferred from the colony-forming potential) (Fig. 4). Like in the above examples, simultaneous exposure to DETC and B-DNIC-GSH decreased the viable cell portion to 5%. All cells died when DETC was added 40 min after adding B-DNIC-GSH. B-DNIC-GSH entered cells in this case, and all nitrosonium cations released from B-DNIC-GSH upon its interaction with DETC were released within cells to exert their cytopathogenic effect.
Effects of DETC and B-DNIC-GSH on the colony-forming activity of E. coli TN350 cells. Columns: (1) incubation with 2.5 mM DETC; (2) incubation with 0.5 mM B-DNIC-GSH; (3) sum of the DETC and B-DNIC-GSH effects (columns 1 + 2) in the absence of their interaction; (4) simultaneous exposure to DECT + B‑DNIC-GSH; 5, B-DNIC-GSH was added 40 min after adding DETC [24].
As was observed recently, superoxide anion radicals or divalent iron-chelating agents can be used to decompose DNICs with thiol-containing ligands with a release of nitrosonium cations. Superoxide anions react within DNICs with NO molecules, which occur as nitrosyl ligands in IDNMs of the complexes [25]. It was already mentioned above that half of the ligands occurs as neutral NO molecules in DNIC resonance structures, while the other half occurs as nitrosonium cations. When superoxide anions interact with NO molecules, peroxinitrite forms in the ligand sphere of iron in DNICs and is then isomerized to nitrate within DNICs, and DNICs are consequently decomposed to release nitrosonium cations. As for the decomposing effect of iron-chelating agents, DNICs are decomposed indeed [26] and nitrosonium cations are released during their decomposition. The release takes place apparently because all ligands are replaced with iron-chelating agent molecules in the surrounding of bivalent iron in DNICs. This mechanism might be responsible for the cytotoxic effect that B-DNIC-GSH exerted on HeLa cells in the presence of the iron-chelating agent bathophenanthroline disulfonate or ethylenediaminetetraacetate (EDTA) [27].
It was found that SARS-CoV-2 replication in model animals (Syrian hamsters) is possible to suppress by taking advantage of the release of nitrosonium cations as cytotoxic agents from B-DNIC-GSH and its dramatic increase in the presence of DETC. The finding opens the opportunity to design drugs against COVID-19 on the basis of DNICs with thiol-containing ligands and dithiocarbamate derivatives (or DNICs alone possibly).
Experiments with Syrian hamsters infected with SARS-CoV-2 were carried out in the Vector State Research Center of Virology and Biotechnology (Kol’tsovo, Novosibirsk region). Hamsters were kept in a closed chamber, and B-DNIC-GSH and DETC solutions were sprayed consecutively into the air supplied into the chamber (B-DNIC-GHS for 30 min and then, after 30 min, DETC for 30 min). Both B‑DNIC-GSH and DETC were used at 10 mM; 10 mL of each solution was added into a sprayer. Treatments with the compounds were performed twice daily for 4 days. The coronavirus-infected Syrian hamsters were then sacrificed. Nasal and lung samples were collected, homogenized, and tested for virus RNA amount. The RNA amount was determined by RT-qPCR (using the number of cycles Ct) and titration in cultured Vero 6 cells (lgTCID50/mL) [28].
Viral loads established in the Syrian hamsters by RT–qPCR are summarized in Table 1. Used alone, B‑DNIC-GSH administered as a spray significantly decreased the viral load only in nasal cavity tissues, where the load decreased by a factor of 16 as compared with control hamsters (treated with placebo). Measurements of the infectious titer (logTCID50/mL) did not detect a significant decrease in viral load in nasal or lung tissue.
A higher therapeutic effect was observed in hamsters treated consecutively with B-DNIC-GSH and DETC. The RNA accumulation in nasal tissues decreased by a factor of 16 according to the RT–qPCR results, like in the case of B-DNIC-GSH used alone. However, a therapeutic effect was detected in lung tissue as well; i.e., the virus RNA load decreased by a factor of 20 as compared with the placebo group. Virus titration showed a greater treatment efficacy. The virus titer in nasal tissues decreased by a factor of 200 as compared with the placebo group, while a decrease observed in lung tissues was much the same as reported by RT-qPCR (by a factor of 20).
When infected hamsters were treated with DETC alone or with DETC followed by B-DNIC-GSH, both administered as sprays, a therapeutic effect was totally absent with this administration regimen.
It is of interest that EPR did not detect the formation of MNIC-DETC by the characteristic triplet signal with a center at g = 2.04 after consecutive administration of B-DNIC-GSH and DETC to hamsters, even in the lung [28]. MNIC-DETC was expected to result from the interaction of B-DNIC-GSH with DETC according to Scheme 4 to release nitrosonium cations from B-DNIC. Nitrosonium cations were assumed to act as a cytotoxic agent and to exert a therapeutic effect by suppressing SARS-CoV-2 replication in the infected hamsters.
The failure to detect MNIC-DETC in the lung was most likely explained by the destructive effect of superoxide on MNIC-DETC. The effect was demonstrated [29] and explained by the binding of the superoxide anion with an NO molecule in MNIC-DETC. The reaction was already considered above when discussing DNIC decomposition by superoxide anions. Note that the superoxide anion level is probably high in the lung, which is in immediate contact with air and oxygen as its component, at least high enough to destroy MNIC-DETC.
The capability of S-nitrosylating thiols, including both low-molecular-weight and protein ones, is the main property of nitrosonium cations that determines their functional role in living organisms. Therefore, there are grounds to think that S-nitrosylation of various virus proteins (proteases, capsid proteins, reverse transcriptases, and transcription factors) and host proteases by nitrosonium cations was responsible for a decrease in virus production in the host organism, providing an efficient means to protect animals and humans from virus infection, including SARS-CoV-2 infection.
The question arises as to whether it is possible to obtain DNICs with thiol-containing ligands that release nitrosonium cations in sufficient amounts without being decomposed by other agents, such as dithiocarbamate derivatives, and thus exert the antivirus effect to treat, for example, COVID-19.
This is possible in fact. However, such processes are possible in DNIC solutions with a lower content of free (not included in DNICs) thiols. This is evident from the following experimental data (A.F. Vanin, prepared for publication). An original method was developed to synthesize DNICs with thiol-containing ligands according to Scheme 2. The method was used to obtain the B-DNIC-GSH preparations with free glutathione : B-DNIC-GSH ratios of 2 : 1 and : 0 : 1. The preparations were used to check the property observed for B-DNIC-GSH earlier, namely, that nitrosonium cations are released from B-DNIC-GSH in a highly acidic medium (pH 1–2) at 80°C on exposure to air [30].
Both of the preparations were found to release nitrosonium cations in the above conditions with similar efficiencies. Nitrosonium cations were detected by the formation of GS-NO, which is a characteristic product of their binding with GSH and has optical absorption maximums at 334 and 540 nm (Fig. 1). However, NO+ might appear in the medium upon B‑DNIC-GSH decomposition in aerobic conditions not only because NO+ is released from the complexes, but also because NO is released and oxidized to nitrogen dioxide (NO2) with subsequent generation of dinitrogen trioxide (N2O3), which is capable of S‑nitrosylating thiols. To check this possibility, similar experiments with the B-DNIC-GSH solutions were carried out in anaerobic conditions, in the absence of air. Only the B-DNIC-GSH preparations where all glutathione molecules were included in complexes (i.e., a free glutathione : B-DNIC-GSH molar ratio was 0 : 1) were found to release nitrosonium cations in these conditions. The nitrosonium ion concentration was even greater than the B-DNIC concentration (Fig. 5a, curve 2). The concentration might be higher because GS-NO molecules were not all included in B‑DNIC-GSH during its formation. The molecules that had remained free could contribute to the GS-NO pool released from B-DNIC. The assumption agrees with the fact that the B-DNIC-GSH level in this case was lower than in the solutions obtained using a twice higher free glutathione concentration relative to B-DNIC (Figs. 5b, 5c; curves 1).
Evolution of B-DNIC-GSH absorption spectra. B-DNIC-GSH was synthesized at MS : Fe2+: nitrite ratios of (a) 20 : 20 : 20 or (b, c) 40 : 20 : 20 mM. The preparations were then acidified to pH 1.0 and incubated at 80°C in (a, b) anaerobic or (c) aerobic conditions. Curve 1, initial B-DNIC-GSH solution. (a, b) Curves 2, 3, the solution was tested, respectively, 5 min after being heated at pH 1.0 and then supplemented with an excess of glutathione. (c) Curves 2‒4, the solution was tested, respectively, 1 min after being acidified, heated at 80°C for 1 min, and supplemented with an excess of glutathione. The B-DNIC-GSH solutions were diluted 20 fold to record the spectra. (c) Vertical lines are at 330 and 334 nm.
In the solutions where glutathione was used as a twice higher concentration than the complex, the complex decomposed without appreciable GS-NO production (Fig. 5b). When the same solution was heated at acidic pH, but in aerobic conditions, optical absorption of GS-NO was detected and the GS-NO concentration was equal to the B-DNIC concentration. Subsequent addition of an excess of glutathione increased the GS-NO concentration (Fig. 5c; curves 3, 4).
The following conclusions and assumptions are possible to make based on the findings. First, nitrosonium cations occur in B-DNIC-GSH in fact and can be released from the complex, but at a low (better, zero) concentration of the free thiol (GSH) in the solution. Second, when the free thiol content is higher in a solution of complexes, nitrosonium cations released from the complexes can be reduced to NO, apparently, by the free thiol. Thiols alone are incapable of this reduction because the law of spin conservation in chemical reactions would be violated otherwise. The process is possible when iron ions are involved to act as spin catalyzers [31]. The assumption that all nitrosyl ligands are released in the form of NO from B-DNIC-GSH at a free glutathione : B-DNIC-GSH ratio of 2 : 1 is supported by the results of measuring NO released from the respective DNIC solutions in the gaseous phase [11].
A release of NO+ was observed in B-DNIC-GSH solutions, but was incomplete, when the free GSH : B-DNIC ration was 1 : 1 [11]. It was observed in the same study that the NO+ yield drops to zero when the ratio is increased to 2 : 1.
When solutions with a GSH : B-DNIC ratio of 2 : 1 were heated in aerobic conditions, GS-NO generation was detected and its concentration was the same as the complex concentration (Fig. 5c, curve 3), possibly because free GSH was totally oxidized by air oxygen. The GS-NO : B-DNIC-GSH ratio consequently became 0 : 1, at which released nitrosonium cations were not reduced to NO and could be included in GS-NO. An increase in GS-NO after subsequent addition of excess GSH indicates that an additional NO+ amount is generated apparently as a component of dinitrogen trioxide, which is produced in the reaction between NO and NO2, a product of NO oxidation by air oxygen (Fig. 5c, curve 4). Characteristically, an increase in GS-NO was not observed when excess glutathione was added in anaerobic conditions to solutions with a GSH : B-DNIC-GSH ratio of 0 : 1 (Fig. 5a, curve 3).
A cytotoxic effect of nitrosonium cations was for the first time observed in experiments with the bacterium Clostridium sporogenes and cultured Swiss 3T3 fibroblasts [32, 33]. In particular, dose-related cytotoxicity of nitrosonium cations was shown to be 40 times higher than that of NO molecules in fibroblast cultures. Nitroprusside ions [(CN-)5Fe2+NO+]2–, which act as NO+ donors, caused apoptosis in 50% of fibroblasts after 2-h incubation with 20 µM nitroprusside, while a comparable effect of NO on fibroblasts was observed at an NO concentration of 800 µM after 24-h incubation with gaseous NO or NO donors (GS-NO or S-nitroso-N-acetylpenicillamine (SNAP) [33]. In experiments with C. sporogenes, red and black Roussin’s salts were used as putative donors of nitrosonium cations. However, it was not demonstrated unambiguously that NO+ cations, rather than NO molecules, are released from the complexes to mediate their cytotoxic effect [32]. This was demonstrated for the cytotoxic effect of M-DNIC-TS on Jurkat cells [21], which showed a synergistic effect for M-DNIC-TS and MGD used together. Further studies confirmed that this approach is reasonable to use in the case of DNICs with thiol-containing ligands in order to demonstrate that nitrosonium cations, rather than NO molecules, are responsible for their cytotoxicity.
The above conclusion gives grounds to state that a proapoptotic effect of DNICs with thiol-containing ligands in HeLa cells [27] and their inhibitory effect on endometrioma cell proliferation in rats with experimental endometriosis [34, 35] should also be considered as examples of the cytotoxic effect on nitrosonium cations on biosystems. The same is possible to say about the findings that DNICs with cysteine suppress fibrosis of the denervated penile corpora cavernosa in rats [36] and B-DNICs with mercaptoethanol exert a similar effect in kidney fibrosis induced by ligation of the ureter [37].
It cannot be excluded that a process that naturally sustains cell immunity in humans and animals is simulated by using DNICs with thiol-containing ligands as donors of nitrosonium cations, which exert a cytotoxic effect on cells and tissues. The only difference is that endogenous DNICs with thiol-containing ligands are utilized as a working form of nitric oxide in nature, while the same, but exogenous complexes are chemically synthesized in vitro and utilized in research. It this is true and endogenous DNICs with thiol-containing ligands are used indeed by the immune system to eliminate malignant cells and tissues in animals and humans, then exogenous DNICs will probably allow a substantial progress in the field of cancer treatment. The effects of B-DNICs with thiol-containing ligands on tumor cell proliferation has been studied in mouse tumor graft models by my team for several years. Suppression of tumor cell proliferation is a promising finding [38, 39].
As compounds that act like a working form of endogenous nitric oxide, exogenous DNICs with thiol-containing ligands fully simulate the positive regulatory effects of endogenous nitric oxide (better to say, the endogenous nitric oxide system) in humans and animals. A higher dose-dependent efficiency is characteristic of exogenous DNICs compared with gaseous NO as concerns, for example, their vasodilator and wound-healing activities. To illustrate the vasodilator effects, Fig. 6 shows dose-dependent dilation of isolated circular segments of the rat aorta in response to gaseous NO and M-DNICs with cysteine or acetylcholine (Ach) [40].
Vasodilator effects of (a) NO, (b) M-DNIC with cysteine, and (c) acetylcholine (Ach) in the absence or presence of superoxide dismutase (–SOD and +SOD, respectively). The effects were studied in isolated ring rat aorta segments (with the endothelium preserved), which were preliminarily constricted with norepinephrine (10–7 M). Dots show the time points of adding the vasodilators (logM), atropine (Atr, 10–5 M), or hemoglobin (Hb, 10–5 M). Vertical bar, 1 g; horizontal bar, 5 min [40].
As is seen from Fig. 6, the dose-related efficiency of M-DNIC with cysteine was much the same as that of the endogenous vasodilator acetylcholine. Hemoglobin, which acts as a NO scavenger, abolished the vasodilator effects of DNICs and acetylcholine. Superoxide dismutase (SOD), which decreases the superoxide level and thus prevents superoxide-dependent NO elimination, increased the vasodilator effects of DNICs and acetylcholine. A similar increase was observed in the vasodilator effect of gaseous NO in the presence of SOD. The NO effect observed in this case approached the effect that DNICs exerted in the absence of SOD. This means that incorporation in DNICs stabilized NO (protected NO from superoxide), like SOD similarly protected free NO molecules. Atropine stabilized the effect of acetylcholine.
The dose dependence of the vasodilator effect in the absence of SOD was compared in detail between NO and DNICs. The comparison showed that incorporation of 10–8 ‒10–7 M NO in DNIC with cysteine increased the dose-related vasodilator effect of NO by a factor of 100‒300, apparently suggesting protection of NO from superoxide [40]. According to the data reported in [26], the protective effect most likely arises because superoxide binds with NO in IDNM of DNIC and peroxinitrite formed in the complex is then isomerized to nitrate. Thus, DNICs can act as superoxide scavenger, that is, antioxidants.
When the wound-healing effect was compared for NO and B-DNIC-GSH, the same efficiencies of the agents were achieved at a B-DNIC concentration that was 25 times lower than the respective NO concentration [41].
High vasodilator activity of DNICs with thiol-containing ligands underlies their efficient hypotensive action in animals and humans [42, 43]. It was therefore of interest to compare hypotensive activity between gaseous NO and DNICs. Naturally, gaseous NO was administered and delivered into the blood through lung airways. Appreciable changes in blood pressure were not observed, although NO delivery into the blood was confirmed by detecting nitrosyl complexes of hemoglobin by EPR [44]. Why a hypotensive effect of NO was lacking (although NO, like oxygen, should be delivered to vascular walls by hemoglobin)? The question is still open. As for a hypotensive effect of DNICs with thiol-containing ligands, their intravenous administration was found to substantially decrease the blood pressure in humans. A 20% decrease in blood pressure was achieved at a B‑DNIC-GSH dose of 0.2 µmol/kg body weight in humans and 1 µmol/kg in rats [43].
To supplement the above data on the regulatory effects of DNICs with thiol-containing ligands, other effects reported for DNICs as NO donors to date are summarized in Table 2.
The list in Table 2 will certainly be greatly expanded, given that virtually all vital processes involve the endogenous nitric oxide system and, therefore, DNICs with thiol-containing ligands as its working form.
REFERENCES
Ignarro L.J. 2000. Nitric Oxide Biology and Pharmacology. Zurich: Academic.
Domingos P., Prado A.M., Wong A., Gehring C., Feijo J. 2015. Nitric oxide: A multitasked signaling gas in plants. Mol. Plant. 8, 506‒520.
Stern A., Zhu J. 2014. An introduction to nitric oxide sensing and response in bacteria. Adv. Appl. Microbiol. 87, 187‒220.
Gaston B.M., Carver J., Doctor A., Palmer L.A. 2003. Physiological roles of S-nitrosylation. Mol. Interventions. 3, 253‒263.
Seth D., Hess D.T., Hausladen A., Wang L.W., Wang A.J., Stamler J.S. 2018. A multiplex enzymatic machinary for cellular protein S-nitrosylation. Mol. Cell. 89, 451‒464.
Vanin A.F. 2015. Dinitrozil’nye kompleksy zheleza s tiol-soderzhashchimi ligandami (Fiziko-khimiya, biologiya, meditsina). (Iron Dinitrosyl Complexes with Thiol-Containing Ligands (Physical Chemistry, Biology, and Medicine)). Izhevsk: Institut komp’yuternykh issledovanii.
Lu T.T., Wang Y.M., Hung C.H., Chiou S.J., Liaw W.F. 2018. Bioinorganic chemistry of the natural [Fe(NO)2] motif evolution of a functional model for NO-related biomedical application and revolutionary development of a translation model. Inorg. Chem. 57, 12425‒12443.
Vanin A.F. 2019. Dinitrosyl Iron Complexes as a “Working Form” of Nitric Oxide in Living Organisms. Cambridge, UK: Cambridge Scholar Publ.
Lehnert N., Kim E., Dong H.T., Harland J.B., Hunt A.H., Manikas E.C., Oakley K.M., Pham J., Reed G.C., Alfaro V.S. 2021. The biologically relevant coordination chemistry of iron and nitric oxide: Electronic structure and reactivity. Chem. Rev. 121, 14682‒14905.
Vanin A.F., Burbaev D.S. 2011. Electronic and spatial structures of water-soluble dinityrosyl iron complexes with thiol-containing ligands underlying their activity to act as nitric oxide and nitrosonium ion donors. Biophys. J. 2011, 878236.
Vanin A.F. 2020. How is nitric oxide (NO) converted into nitrosonium cations (NO+) in living organisms? (Based on the results of optical and EPR analysis of dinitrosyl iron complexes with thiol-containing ligands) Appl. Magn. Res. 51, 851‒876.
Vanin A.F. 2020. The free-radical nature of nitric oxide molecules as a determinant of their conversion to nitrosonium cations in living systems. Biophysics (Moscow). 65, 353‒377.
Vanin A.F., Malenkova I.V., Serezhenkov V.A. 1997. Iron catalyzes both decomposition and synthesis of S‑nitrosothiols. Nitric Oxide Biol. Chem. 1, 191‒203.
Vanin A.F. 2023. Research into dinitrosyl iron complexes in living organisms through EPR as an example of applying this method in biology: A review. Appl. Magn. Res. 54, 289‒308.
Vanin A.F., Nalbandyan R.M. 1965. New type of free radicals in yeast cells. Biofizika. 10, 167–168.
Vithaythil A.J., Ternberg J.L., Commoner B. 1965. Changes in electron spin resonance signals of rat liver during chemical carcinogenesis. Nature. 207, 1246‒1249.
Vanin A.F., Poltorakov A.P., Mikoyan V.D., Kubrina L.N., Burbaev D.S. 2011. Polynuclear water-soluble dinitrosyl iron complexes with cysteine or glutathione ligands: Electron paramagnetic resonabnce and optical studies. Nitric Oxide Biol. Chem. 23, 136‒149.
Williams D.L.H. 2004. Nitrosation Reactions and the Chemistry of Nitric Oxide. Amsterdam: Elsevier.
Ascenzi P., diMesi A., Sciore A.C., Clementi E. 2010. Peroxynintrite—an ugly biofactor? Biofactors. 36, 264‒273.
Radi R. 2018. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U. S. A. 115, 5839‒5848.
Kleschyov A.L., Strand S., Schmitt S., Gottfried D., Skatchov M., Sjakste N., Deiber M., Umansky V., Münzel T. 2006. Dinitrosyl-iron triggers apoptosis in Jurkat cells despite overexpression of Bcl-2. Free Radical Biol. Med. 40, 1340‒1348.
Vanin A.F., Tronov V.A., Borodulin R.R. 2021. Nitrosonium cation as a cytrotoxic component of dinitrosyl iron complexes with thio-containing ligands (based on the experimental work on MCF-7 human breast cancer cell culture) Cell. Biochem. Biophys. 79, 93‒102.
Galagan M.E., Oranovskaya E.V., Mordvintsev P.I., Medvedev O.S., Vanin A.F. 1988. Hypotensive effect of iron dinitrosyl complexes on awake animals. Byull. Vses. Kardiol. Tsentra. 2, 75‒79.
Vanin A.F., Telegina V.I., Mikoyan V.D., Tkachev N.A., Vasilieva S.V. 2022. The cytostatic action of dinitrosyl iron complexes with glutathione on Escherichia coli cells is mediated by nitrosonium cations released from these complexes. Biophysics (Moscow). 67, 761‒767.
Vanin A.F., Mikoyan V.D., Tkachev N.A. 2022. Nitrosonium cation release from dinitrosyl iron complexes in the decomposition induced by superoxide anions or ethylenediaminetetraacetate. Biophysics (Moscow). 67, 847‒855.
Shumaev K.B., Gubkin A.A., Serezhenkov V.A., Lobysheva I.I., Kosmachevskaya O.V., Ruuge E.K., Lankin V.Z., Topunov A.F., Vanin A.F. 2008. Interaction of reactive oxygen and nitrogen species with albumin and methemoglobin-bound dinitrosyl iron complexes. Nitric Oxide Biol. Chem. 18, 37‒46.
Giliano N.V., Konevega L.V., Noskin L.A., Serezhenkov V.A., Poltorakov A.P., Vanin A.F. 2011. Dinitrosyl iron complexes with thiol-containing ligands and apoptosis: studies with HeLa cell culture. Nitric Oxide Biol. Chem. 24, 151‒159.
Shipovalov A.V., Vanin A.F., Pyankov O.V., Bagryanskaya E.G., Mikoyan V.D., Tkachev N.A., Asanbaeva N.A., Popkova V.A. 2022. Antiviral activity of nitrosonium cation against SARS-CoV-2 on a Syrian hamster model. Biophysics. 67, 785‒789.
Vanin A.F., Huisman A., Stroes E.S.G., de Ruuter-Huijstek F.C., Rabelink T.J., van Faassen E.E. 2001. Antioxidant capacity of mononitrosyl–iron–dithiocarbamate complexes: Implications for NO trapping. Free Radical Biol. Med. 30, 813‒824.
Vanin A.F. 2018. Nitrosonium ions as constituents of dinitrosyl iron complexes with glutathione responsible for their S-nitrosating activity. Austin J. Analyt. Pharm. Chem. 5, 1109‒1125.
Buchachenko A.L., Berdinsky V.L. 1996. Spin catalysis in chemical reactions. J. Phys. Chem. 100, 18292‒18299.
Gui X., Joannou C.L., Huges M.N., Cammack R. 1992. The bactericidal effects on transition metal complexes containing NO+ group on the food-spoilage bacterium Clostridium sporogenes. FEMS Microbiol. Lett. 98, 67‒70.
Khan S., Kayahara M., Joashi U., Mazarakis N.D., Sarraf C., Edwards A.D., Huges M.N., Mehmet H. 1997. Differential induction of apoptosis in Swiss 3T3 by nitric oxide and the nitrosonium cation. J. Cell Sci. 110, 2315‒2322.
Vanin A.F., Burgova E.N., Adamyan L.V. 2015. Dinitrosyl iron complexes with glutathione suppress surgically induced experimental apoptosis in rats. Austin J. Reprod. Med. Infertil. 2, 1109‒1032.
Burgova E.N., Khristidi Y.L., Kurkov F.V., Mikoyan V.D., Shekhter A.B., Vanin A.F. 2019. The inhibiting effect of dinitrosyl iron complexes with thiol-containing ligands on the growth of endometrioid tumours in rats with experimental endometriosis. Cell. Biochem. Biophys. 77, 69‒77.
Veliev E.I., Kotov S.V., Shishlo V.K., Serezhenkov V.A., Losinsky V.I., Vanin A.F. 2008. Beneficial effect of dinitrosyl iron complexes with thiol ligands on the rat penile cavernous bodies. Biophysics (Moscow). 53, 153‒157.
Lee T.Y., Lu H.H., Chang H.T., Huang H.C., Tsai Y.J., Chang I.H., Tu C.P., Chung C.U., Lu T.T., Peng C.H., Chen Y. 2023. Delivery of nitric oxide with a pH-responsive nanocarrier for the treatment of renal fibrosis. J. Controlled Release. 354, 417‒428.
Vanin A.F., Ostrovskaya L.F., Korman D.B., Mikoyan V.D., Kubrina L.N., Borodulin R.R., Fomina M.M., Rykova V.A. 2015. An antinitrosative system as a factor in malignant tumor resistance to the cytotoxic effect of nitrogen monooxider Biophysics (Moscow). 60, 121‒125.
Vanin A.F., Ostrovskaya L.A., Korman D.B., Bluhterova N.V., Rykova V.A. Fomina M.M. 2022. Role of nitrosonium cation in the mechanism underlying the antitumor effects of drugs in combination with dinitrosyl iron complexes. Biophysics (Moscow). 67, 808‒813.
Vedernikov Y.P., Mordvintcev P.I., Malenkova I.V., Vanin A.F. 1992. Similarity between the vasorelaxing activity of dinitrosyl iron cysteine complexes and endothelium-derived relaxing factor. Eur. J. Pharmacol. 211, 313‒317.
Igrunkova A., Fayzullin A., Serejenkova N., Lipina T., Pekshev A., Vanin A., Zaborova V., Budanova E., Shestakov D., Kastyro I., Shekhter A. 2023. Beneficial effect of dinitrosyl iron complexes on wound healing compared to commercial nitric oxide plasma generator. Int. J. Mol. Sci. 24, 4439–4457.
Kleshchev A.L., Mordvintsev P.I., Vanin A.F. 1985. The role of nitric oxide and iron in the hypotensive effect of iron nitrosyl complexes with various anionic ligands. Studio Biophys. 105, 93‒102.
Chazov E.I., Rodnenkov O.V., Zorin A.V., Lakomkin V.L., Gramovich O.V., Vyborov O.V., Dragnev A.G., Timoshin A.A., Buryachkovskaya L.I., Abramov A.A., Massenko V.P., Arzamastsev E.V., Kapelko V.I., Vanin A.F. 2012. Hypotensive effect of “Oxacom” containing a dinitrosyl iron complexes with glutathione: Animal studies and clinical trials on healthy volunteers. Nitric Oxide Biol. Chem. 26, 148‒157.
Vanin A.F., Pekshev F.V., Vagapov F.V., Sharapov N.A., Lakomkin V.L., Abramov AA., Timoshin A.A., Kapelko V.I. 2021. Gaseous nitric oxide and dinitrosyl iron complexes with thiol-containing ligands as potential medicines that can relieve COVID-19. Biophysics. 66, 155‒163.
Mordvintcev P.I., Rudneva V.G., Vanin A.F., Schimkevich L.L., Khodorov B.I. 1986. Inhibitory effect of dinitrosyl complexes of non-heme iron with low molecular weight ligands on platelet aggregation. Biokhimiya. 51, 1851‒1857.
Shamova E.V., Bichan O.D., Drozd E.S., Gorudko I.V., Chizhik S.A., Shumaev K.B., Cherenkevich S.N., Vanin A.F. 2011. Regulation of the functional and mechanical properties of platelet and red blood cells by nitric oxide donors. Biophysics (Moscow). 56, 237‒242.
Remizova M.I., Kochetygov N.I., Kerbout K.A., Lakomkin V.L., Timoshin A.A., Burgova E.N., Vanin A.F. 2011. Effect of dinitrosyl iron complexes with glutathione on hemorrhagic shock followed by saline treatment. Eur. J. Pharmacol. 662, 40‒36.
Andreev-Andriyevsky A.A., Mikoyan V.D., Serezhenkov V.A., Vanin A.F. 2011. Penil erective activity of dinitrosyl iron complexes with thiol-containing ligands. Nitric Oxide Biol. Chem. 24, 217‒223.
Serezhenkov V.A., Kalinina E.V., Glazunova V.A., Saprin A.N., Vanin A.F. 2007. Why does iron abrogate the cyotoxic effect of S-nitrosothiols on human and animal cultured cells? Biofizika. 52, 869‒875.
Kim Y.M., Chung H.T., Symmons R.L., Billiar T.R. 2000. Cellular nonheme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibitor. J. Biol. Chem. 275, 10954‒10961.
Ding H., Demple B. 2000. Direct nitric oxide signal transduction via nitrosylation of iron−sulfur centers in the SoxR transcription activation. Proc. Natl. Acad. Sci. U. S. A. 97, 5146‒5150.
Lo F.C., Chen C.L., Lee C.M., Tsai M.C., Lu T.T., Liaw W.F., Yu S.S.F. 2008. A study of NO trafficing from dinitrosyl-iron complexes to the recombinant E. coli transcriptional factor SoxR. J. Biol. Inorg. Chem. 13, 961‒972.
Demple B. 2002. Signal transduction by nitric oxide in cellular stress response. Mol. Cell. Biochem. 2345/235, 11‒18.
Wu C.R., Huang Y.D., Hong Y.H., Liu Y.H., Narwane M., Chang Y.H. Dinh N.K., Hsieh H.T., Hsieh Y.J., Wu P.C., Pao C.W., Chan T.S., Hsu I.J., Lu T.T. 2021. Endogenous conjugation of biomimetic dinitrosyl iron complex with protein vehicles for oral delivery of nitric oxide to brain and activation of hippocampal neurogenesis. J. Am. Chem. Soc. Au. 1, 998‒1013.
Lepka K., Volbracht K., Bill E., Schneider R., Rios N., Hildebrandt T., Ingwersen J., Prozorovski T., Lillig C.H., van Horssen J., Steinman L., Har-tung H.P., Radi R., Holmgren A., Aktas O., Berndt C. 2017. Iron-sulfur glutaredoxin 2 protects oligodendrocytes against damage induced by nitric oxide release from activated microglia. Glia. 65, 1521‒1534.
Malyshev I.Yu., Malugin A.V., Golubeva L.Yu., Zenina T.A., Manukhina E.B., Mikoyan V.D., Vanin A.F. 1996. Nitric oxide donor induces HSP70 accumulation in the heart and in cultured cells. FEBS Lett. 301, 21‒23.
Voevodskaya N.V., Serezhenkov V.A., Cooper C.F., Kubrina L.N., Vanin A.F. 2002. Exogenous ferrous irons is required for the nitric oxide-catalyzed destruction of the iron-sulphur center in adrenodoxin. Biochem. J. 368, 633‒639.
Graziano M., Lamattina L. 2005. Nitric oxide and iron in plants: An emerging and converging story. Trends Plant Sci. 10, 4‒8.
Richardson D.R., Lok H.C. 2008. The nitric oxide-iron interplay in mammalian cells: Transport and storage of dinitrosyl iron complexes. Biochim. Biophys. A-cta. 1780, 638‒651.
Pisarenko O.I., Serebryakova L.I., Tskitishvili O.V., Studneva L.M., Vanin A.F., Chazov E.I. 2008. Cardioprotective effect of dinitrosyl iron complexes with cysteine in rats in vivo. Biol. Bull. (Moscow). 35 (1), 95–98.
ACKNOWLEDGMENTS
I am grateful to N. Tkachev for computer design of the figures.
Funding
This work was supported by the Russian Science Foundation (project no. 23-74-00009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This work does not contain any studies involving animals or human participants performed by the author.
CONFLICT OF INTERESTS
The author declares that he has no conflicts of interest.
Additional information
Translated by T. Tkacheva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vanin, A.F. Dinityrosyl Iron Complexes with Thiol-Containing Ligands as a Functionally Active “Working Form” of Nitric Oxide System in Living Organisms: A Review. Mol Biol 57, 929–940 (2023). https://doi.org/10.1134/S0026893323060183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323060183