Abstract
One of the main functions of enzyme complexes that constitute electron transport (respiratory) chains of organisms is to maintain cellular redox homeostasis by oxidizing reducing equivalents, NADH and quinol. Cytochrome bd is a unique terminal oxidase of the chains of many bacteria including pathogenic species. This redox enzyme couples the oxidation of ubiquinol or menaquinol by molecular oxygen to the generation of proton motive force, a universal energy currency. The latter is used by the organism to produce ATP, another cellular energy currency, via oxidative phosphorylation. Escherichia coli contains two bd-type oxidases, bd-I and bd-II, encoded by the cydAB and appCB operons, respectively. Surprisingly, both bd enzymes make a further contribution to molecular mechanisms of maintaining the appropriate redox balance in the bacterial cell by means of elimination of reactive oxygen species, such as hydrogen peroxide. This review summarizes recent data on the redox-modulated H2O2-scavenging activities of cytochromes bd-I and bd-II from E. coli. The possibility of such antioxidant properties in cytochromes bd from other bacteria is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Like most prokaryotes, Escherichia coli possesses a branched and flexible electron transport (respiratory) chain [1, 2]. The chain allows a bacterial population to grow and survive under changing environmental conditions, from oxic oxidized to anoxic reduced [3, 4]. It contains two NADH dehydrogenases (NDH-1 and NDH-2), succinate dehydrogenase (SDH), and three terminal oxygen reductases (bd-I, bd-II, and bo3) [5] (Fig. 1). NDH-1 and NDH-2 oxidize NADH by ubiquinone. SDH oxidizes succinate by ubiquinone. bd-I, bd-II, and bo3 oxidize ubiquinol and/or menaquinol by O2. NDH-1, bd-I, bd-II, and bo3 are primary generators of proton motive force [6‒9] that is used for ATP production [10]. Energy conservation is one of the main functions of the chain. Another important function of the chain is maintaining cellular redox homeostasis. The latter is carried out through the oxidation of reducing equivalents, the NADH and ubiquinol/menaquinol pools.
Components of the respiratory electron transport chain of Escherichia coli. Two NADH dehydrogenases (NDH-1 and NDH-2) transfer electrons from NADH to ubiquinone/menaquinone. Succinate dehydrogenase (SDH) transfers electrons from succinate to ubiquinone/menaquinone. Three terminal oxygen reductases (bd-I, bd-II, and bo3) oxidize ubiquinol/menaquinol by molecular oxygen to produce water. NDH-1, bd-I, bd-II, and bo3 are primary generators of proton motive force. NDH-2 and SDH do not produce proton motive force.
A membrane-bound bd-type terminal oxidase is a key enzyme for bacterial adaptation to adverse environmental conditions, such as the presence of antibiotics [11], sulfide [12‒16], nitric oxide [17‒29], peroxynitrite [30, 31], ammonia [32], cyanide [33‒35]. Cryogenic electron microscopy structures of both cytochrome bd-I [36, 37] and cytochrome bd-II [38, 39] were reported. A unified scheme of structural organization and catalytic function of the E. coli bd enzymes is shown in Fig. 2. The bd-I oxidase is composed of four subunits, CydA, CydB, CydX, and CydH (also called CydY). The bd-II enzyme is built by subunits AppC (homolog to CydA), AppB (homolog to CydB), and AppX (homolog to CydX), that is, one less. CydA/AppC carries a quinol binding site (named the Q-loop) and three hemes, one low spin, b558, and two high spin, b595, and d [40]. Heme b558 is located close to the Q-loop and directly involved in the oxidation of ubiquinol and/or menaquinol [33]. Heme d is the site at which O2 is bound with a high affinity and reduced by four electrons to 2H2O [41]. In E. coli, cytochrome bd-I and cytochrome bd-II are encoded by the cydAB and appCB operons, respectively [4].
Overview of structure and catalytic function of E. coli bd oxidase in the membrane bilayer. Cytochrome bd-I is composed of four subunits, CydA, CydB, CydX, and CydH. Cytochrome bd-II consists of three subunits, AppC, AppB, and AppX. CydA/AppC carries a ubiquinol/menaquinol (QH2) binding site and three hemes, b558, b595, and d. Electrons are transferred from QH2 to heme b558, then to heme b595 and eventually to heme d. The latter is the active site at which O2 is reduced to 2H2O by four transferred electrons and four protons arrived from the inner side of the membrane via a proposed transmembrane proton half channel. The enzyme-catalyzed oxygen reduction reaction is inhibited by NO.
Reactive oxygen species (ROS) include superoxide anion (\({\text{O}}_{2}^{{\centerdot - }}\)), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and singlet oxygen (1O2). They are produced by the host immune system in response to microbial invaders. Bacteria can also generate H2O2 to eliminate their neighbors. E. coli cells growing exponentially produce H2O2 at rates of 9‒22 μM/s [42]. Increased levels of ROS disrupt the cellular redox homeostasis, cause the oxidative stress and severe damage to DNA, RNA, proteins and lipid membranes. Bacteria contain special enzymes, such as catalases, superoxide dismutases and peroxidases, which detoxify ROS [43]. E. coli for this purpose uses the KatG and KatE catalases [44], the NADH peroxidase AhpCF [45], and the cytochrome c peroxidase YhjA [46]. Recently, evidence has emerged that a bd‑type terminal oxidase also contributes to the antioxidant defense machinery in E. coli cells.
CATALASE-LIKE ACTIVITY OF TERMINAL OXIDASES bd-I AND bd-II FROM E. coli
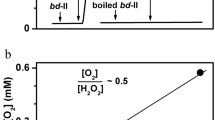
The polarographic studies showed that both cytochrome bd-I and cytochrome bd-II, being isolated from the E. coli membranes without tags, decompose H2O2 with concomitant evolution of O2 [47‒49]. The fact that O2 formation is no longer observed after thermal inactivation of the cytochrome suggests that this activity is associated with a native protein rather than adventitious transition metals in the enzyme preparation. Approximately half a mole of O2 is evolved per mole of H2O2 added. The reaction product, O2, apparently does not inhibit the catalytic decomposition of H2O2 because the reaction rates under microaerobic and aerobic conditions are virtually identical. The rate of O2 evolution increases linearly with enzyme concentration. The reaction also accelerates linearly with the H2O2 concentration until the latter reaches 0.5 mM. At higher concentrations of H2O2 the rate tends to saturate. The apparent kcat and KM are about 165 s–1 and 1.7 mM for cytochrome bd-I and 1030 s–1 and 3.9 mM cytochrome bd-II, respectively. Both enzymes retain the catalase-like activity when they are in turnover with the reducing substrate ubiquinol-1 and the terminal oxidant O2. The fact that H2O2 does not inhibit the O2 reductase activity in which heme d is directly involved indicates that the participation of this heme in the catalase-like reaction is unlikely.
Remarkably, the catalase-like activity of both cytochromes in the presence of excess ubiquinol-1 is no longer observed after they consume all O2 in the measuring chamber and are converted into the fully reduced state. If a commercial catalase is then added to the chamber as a control, the О2 production is observed again. Thus, this activity is sensitive to the enzyme redox state. This finding can exclude the possibility that the observed activity is due to impurities in the enzyme preparations which contain an E. coli catalase. It is unlikely that the contaminant catalase, if present, would be redox-sensitive. It is known that usually dithionite, a very potent reductant, cannot reduce a catalase [50], even if a redox mediator is added [51]. Furthermore, the catalase-like activity is also observed in vivo [47]. There are significant rates of О2 evolution following the addition of H2O2 to respiring E. coli UM2 cells which are deficient in the KatG and KatE catalases but overexpressing cytochrome bd-I. If the bd-I enzyme is not overexpressed, the activity is not observed.
It should be noted that neither the isolated hexa-histidine-tagged bd-I enzyme and nor membrane fractions with Strep-tagged cytochrome bd-I lacking CydH reveal a catalase-like activity [52, 53]. For production of the His6-tagged bd-I protein, a cydABX pACYC177 overexpression vector modified by addition of six histidine triplets at the 3′-end of cydA (C‑terminal His6-tag) was used [52]. For production of the Strep-tagged cytochrome bd-I, a pET17bpcydABX vector with a linker and streptag-II sequence down-stream of cydX was used [53]. The data reported in [52, 53] are inconsistent with our results on the untagged bd-type oxidases [47‒49]. This discrepancy could be due to the differences between the enzyme forms, untagged vs tagged, and other experimental conditions. Additional work is required to resolve the discrepancy.
To try to identify a specific site in the enzyme that is associated with the catalase-like activity, we checked a few inhibitors with different mode of action. We found that both CO and NO, which were reported to target the ferrous heme d [17, 54], do not inhibit the activity. This further suggests that heme d is not responsible for the reaction. N-ethylmaleimide was found to be ineffective to inhibit the activity meaning that a sulfhydryl group is not involved in the activity. Antimycin A and the oxidized ubiquinone-1 also do not affect this activity suggesting that the quinol binding site of the enzymes does not participate in the reaction. Cyanide and azide, on the contrary, turned out to inhibit the catalase-like activity of both cytochrome bd-I and cytochrome bd-II. Both usually target a high-spin ferric heme. The apparent IC50 for NaCN are 2.5 and 4.5 µM for the bd-I and bd-II, respectively. 100 µM NaN3 was reported to inhibit 98% and 35% of the activity of the bd-I and bd-II, respectively. Cyanide and azide are much poorer inhibitors of the O2 reductase activity of the enzymes and most likely do it via binding to the high-spin heme d [4, 33, 55]. In view of the latter fact, much higher sensitivity of the catalase-like activity to these poisons points to the involvement of a high-spin heme, different from heme d, in the reaction. Since a bd-type oxidase contains only two high-spin hemes, b595 and d, the former could be a reasonable candidate (Fig. 3). In this regard, it is interesting to note that the reduced-minus-oxidized difference absorption spectrum of heme b595 is similar to those of catalases and peroxidases which contain protoheme IX as a cofactor [56]. The addition of 50 µM NaCN, completely inhibiting the catalase-like activity, gives rise to a red shift in the Soret absorption band in both enzymes. The magnitudes of the induced absorption change suggest that the inhibitor binds to a high-spin b-type heme in a small fraction of the cytochrome population [47, 49]. This in turn might indicate that only a minor part of the enzyme macromolecules participates in the reaction. Further experiments are required to test this hypothesis.
PEROXIDASE-LIKE ACTIVITY OF TERMINAL OXIDASE bd-I FROM E. coli
The E. coli cytochrome bd-I is also able to scavenge H2O2 via peroxidase-like mechanism that was first observed with the untagged purified enzyme [57]. The electron donors, such as guaiacol, ferrocene, benzohydroquinone, and ferrocyanide (at the ratio of ferrocyanide to ferricyanide of 1 : 10) were shown to be oxidized by the enzyme under aerobic conditions following the addition of H2O2. The effect of several inhibitors of the O2 reductase activity on the peroxidase-like activity of cytochrome bd-I was examined using guaiacol as the substrate. Cyanide, 2-n-heptyl 4‑hydroxyquinoline-N-oxide (HQNO), and pentachlorophenol appeared to inhibit the enzyme-mediated peroxidation of guaiacol [57]. This finding suggests that guaiacol reacts with the enzyme at the level of the quinol binding site and donates electrons to heme d via heme b558 located close to the site and probably heme b595. The electrons reduce the H2O2 bound to heme d to form water (Fig. 4). In these experiments, the rate of the peroxidase-like reaction was quite low, ∼4 s–1. Nevertheless, it was suggested that the activity should be significantly higher in vivo conditions in which the endogenous ubiquinol-8 and/or menaquinol-8 serves as the substrate [31].
Schematic representation of proposed mechanism of peroxidase-like reaction catalyzed by E. coli oxidase bd-I. The peroxidase-like reaction requires decylubiquinol (QH2) as the electron donor and likely occurs at heme d [52]. The reaction is inhibited by NO.
Later, the peroxidase-like activity of the isolated hexa-histidine-tagged bd-I enzyme under anaerobic conditions was detected [52]. In this work, decylubiquinol was used as the substrate. The kcat and KM for H2O2 were determined to be 101 s–1 and 6.6 mM, respectively, yielding a specificity constant, kcat/KM, of 1.5 × 104 M–1 s–1. Unlike the catalase-like activity [47‒49], peroxidation of decylubiquinol is quickly and reversibly inhibited by NO [52]. The latter further suggests that heme d is directly implicated in the peroxidase-like mechanism. Thus, the peroxidase-like reaction requires decylubiquinol as the electron donor and likely occurs at heme d [52]. HQNO at the concentrations of 10‒15 μM causes 50% inhibition of the decylubiquinol peroxidase activity. Hence, in this reaction decylubiquinol donates electrons directly at the quinol binding site of the enzyme.
The ability of the bd-type terminal oxidase to degrade H2O2 at high rates may play a role in the E. coli physiology by protecting the microbial cells against oxidative stress and maintaining a balanced redox status.
ARE bd-TYPE TERMINAL OXIDASES FROM OTHER BACTERIA CAPABLE OF H2O2 DETOXIFICATION?
To date the H2O2-scavenging activities were clearly demonstrated only for the bd enzymes of E. coli. But do cytochromes bd from other bacteria have these activities? For now, this question remains open. Nonetheless, there is some evidence that such a protective function of the bd oxidase is possibly not limited to E. coli. It was reported that low H2O2 concentrations are significantly more toxic to the Azotobacter vinelandii mutant lacking cytochrome bd than to the wild-type [58]. Brucella abortus mutants deficient in cytochrome bd activity also reveal heightened sensitivity to H2O2 [59]. The sensitivity is reversed in transformants provided with the plasmid pSEK102 having the entire cydAB operon. Overexpression of Cu+2/Zn+2 superoxide dismutase and catalase is sufficient to alleviate the loss of cytochrome bd suggesting similar antioxidant functions of these enzymes in B. abortus. The transcriptome analysis of the cellular response of Staphylococcus aureus to H2O2-induced oxidative stress showed that the cydAB genes are strongly induced upon exposure to this ROS, pointing to the role of cytochrome bd in oxidative protection processes [60]. It was found that in Mycobacterium tuberculosis inactivation of the thioredoxin-like protein Rv3673c, required for heme insertion in cytochrome c, results in both increased expression of the bd oxidase and increased resistance to H2O2 [61]. This finding suggests that the M. tuberculosis cytochrome bd may contribute to enzymatic antioxidative defense mechanisms via direct detoxification of H2O2 [62]. Consistently, Mycobacterium smegmatis mc2155 mutant ΔcydA::kan shows the hypersensitivity to exogenous H2O2, indicating that cytochrome bd plays a protective role during oxidative stress in this bacterium [63]. Exponentially growing Δcydbd mutant strain of Porphyromonas gingivalis was reported to demonstrate the increased susceptibility to H2O2 compared to the wild-type [64]. The complementation of the mutant with the native cydAB genes partially restores the resistance to this ROS. Similarly, Δcydbd mutant strain of Alishewanella sp. WH16-1 appeared to be more sensitive to H2O2 than the wild-type, suggesting that the bd oxidase could catalyze the decomposition of H2O2 [65]. Consistently, in the case of Xanthomonas oryzae pv. oryzicola strain BLS256, cydA- and cydAB-knockout mutants display a higher sensitivity to H2O2 along with a reduced bacterial virulence compared to the wild-type [66]. Summarizing the above, it can be assumed that the bd enzymes in the above-mentioned bacteria also contribute to the molecular mechanisms of protection against ROS-induced oxidative damage through the direct elimination of H2O2.
CONCLUSIONS
Studies of cytochromes bd at the molecular level suggest that the enzymes from E. coli and possibly other bacteria enable the microbial cell to maintain a balanced redox status in two different ways: via (i) the oxidation of the membrane-bound quinol molecules and (ii) ROS detoxification.
REFERENCES
Poole R.K., Cook G.M. 2000. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 43, 165–224.
Kaila V.R.I., Wikstrom M. 2021. Architecture of bacterial respiratory chains. Nat. Rev. Microbiol. 19, 319‒330.
Siletsky S.A., Borisov V.B. 2021. Proton pumping and non-pumping terminal respiratory oxidases: Active sites intermediates of these molecular machines and their derivatives. Int. J. Mol. Sci. 22, 10852.
Borisov V.B., Verkhovsky M.I. 2015. Oxygen as acceptor. EcoSal Plus. 6 (2). https://doi.org/10.1128/ecosalplus.ESP-0012-2015
Borisov V.B., Forte E. 2022. Bioenergetics and reactive nitrogen species in bacteria. Int. J. Mol. Sci. 23, 7321.
Matsushita K., Ohnishi T., Kaback H.R. 1987. NADH-ubiquinone oxidoreductases of the Escherichia coli aerobic respiratory chain. Biochemistry. 26, 7732‒7737.
Puustinen A., Finel M., Haltia T., Gennis R.B., Wikstrom M. 1991. Properties of the two terminal oxidases of Escherichia coli. Biochemistry. 30, 3936‒3942.
Jasaitis A., Borisov V.B., Belevich N.P., Morgan J.E., Konstantinov A.A., Verkhovsky M.I. 2000. Electrogenic reactions of cytochrome bd. Biochemistry. 39, 13800‒13809.
Borisov V.B., Murali R., Verkhovskaya M.L., Bloch D.A., Han H., Gennis R.B., Verkhovsky M.I. 2011. Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode. Proc. Natl. Acad. Sci. U. S. A. 108, 17320–17324.
Zharova T.V., Grivennikova V.G., Borisov V.B. 2023. F1·Fo ATP Synthase/ATPase: Contemporary view on unidirectional catalysis. Int. J. Mol. Sci. 24, 5417.
Seregina T.A., Lobanov K.V., Shakulov R.S., Miro-nov A.S. 2022. Inactivation of terminal oxidase bd-I leads to supersensitivity of E. coli to quinolone and beta-lactam antibiotics. Mol. Biol. (Moscow). 56, 572‒579. https://doi.org/10.1134/S0026893322040100
Forte E., Borisov V.B., Falabella M., Colaco H.G., T-inajero-Trejo M., Poole R.K., Vicente J.B., Sarti P., Giuffre A. 2016. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 6, 23788.
Korshunov S., Imlay K.R., Imlay J.A. 2016. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol. Microbiol. 101, 62‒77.
Forte E., Giuffre A. 2016. How bacteria breathe in hydrogen sulphide-rich environments. Biochemist. 38, 8‒11.
Borisov V.B., Forte E. 2021. Terminal oxidase cytochrome bd protects bacteria against hydrogen sulfide toxicity. Biochemistry (Moscow). 86, 22‒32.
Borisov V.B., Forte E. 2021. Impact of hydrogen sulfide on mitochondrial and bacterial bioenergetics. Int. J. Mol. Sci. 22, 12688.
Borisov V.B., Forte E., Konstantinov A.A., Poole R.K., Sarti P., Giuffre A. 2004. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett. 576, 201‒204.
Borisov V.B., Forte E., Sarti P., Brunori M., Konstantinov A.A., Giuffre A. 2006. Nitric oxide reacts with the ferryl-oxo catalytic intermediate of the CuB-lacking cytochrome bd terminal oxidase. FEBS Lett. 580, 4823‒4826.
Borisov V.B., Forte E., Sarti P., Brunori M., Konstantinov A.A., Giuffre A. 2007. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem. Biophys. Res. Commun. 355, 97‒102.
Forte E., Borisov V.B., Konstantinov A.A., Brunori M., Giuffre A., Sarti P. 2007. Cytochrome bd, a key oxidase in bacterial survival and tolerance to nitrosative stress. Ital. J. Biochem. 56, 265‒269.
Mason M.G., Shepherd M., Nicholls P., Dobbin P.S., Dodsworth K.S., Poole R.K., Cooper C.E. 2009. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat. Chem. Biol. 5, 94‒96.
Borisov V.B., Forte E., Giuffre A., Konstantinov A., Sarti P. 2009. Reaction of nitric oxide with the oxidized di-heme and heme-copper oxygen-reducing centers of terminal oxidases: Different reaction pathways and end-products. J. Inorg. Biochem. 103, 1185‒1187.
Giuffre A., Borisov V.B., Mastronicola D., Sarti P., Forte E. 2012. Cytochrome bd oxidase and nitric oxide: From reaction mechanisms to bacterial physiology. FEBS Lett. 586, 622–629.
Giuffre A., Borisov V.B., Arese M., Sarti P., Forte E. 2014. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim. Biophys. Acta. 1837, 1178–1187.
Shepherd M., Achard M.E., Idris A., Totsika M., Phan M.D., Peters K.M., Sarkar S., Ribeiro C.A., Holyoake L.V., Ladakis D., Ulett G.C., Sweet M.J., Poole R.K., McEwan A.G., Schembri M.A. 2016. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Sci. Rep. 6, 35285.
Holyoake L.V., Hunt S., Sanguinetti G., Cook G.M., Howard M.J., Rowe M.L., Poole R.K., Shepherd M. 2016. CydDC-mediated reductant export in Escherichia coli controls the transcriptional wiring of energy metabolism and combats nitrosative stress. Biochem. J. 473, 693‒701.
Jones-Carson J., Husain M., Liu L., Orlicky D.J., Vazquez-Torres A. 2016. Cytochrome bd-dependent bioenergetics and antinitrosative defenses in Salmonella pathogenesis. mBio. 7, e02052‒02016.
Meng Q., Yin J., Jin M., Gao H. 2018. Distinct nitrite and nitric oxide physiologies in Escherichia coli and Shewanella oneidensis. Appl. Environ. Microbiol. 84, e00559‒e00518.
Beebout C.J., Eberly A.R., Werby S.H., Reasoner S.A., Brannon J.R., De S., Fitzgerald M.J., Huggins M.M., Clayton D.B., Cegelski L., Hadjifrangiskou M. 2019. Respiratory heterogeneity shapes biofilm formation and host colonization in uropathogenic Escherichia coli. mBio. 10 (2), e02400-18.
Borisov V.B., Forte E., Siletsky S.A., Sarti P., Giuffre A. 2015. Cytochrome bd from Escherichia coli catalyzes peroxynitrite decomposition. Biochim. Biophys. Acta. 1847, 182‒188.
Borisov V.B., Forte E., Siletsky S.A., Arese M., Davletshin A.I., Sarti P., Giuffre A. 2015. Cytochrome bd protects bacteria against oxidative and nitrosative stress: A potential target for next-generation antimicrobial agents. Biochemistry (Moscow). 80, 565‒575.
Forte E., Siletsky S.A., Borisov V.B. 2021. In Escherichia coli ammonia inhibits cytochrome bo 3 but activates cytochrome bd-I. Antioxidants (Basel). 10, 13.
Borisov V.B., Gennis R.B., Hemp J., Verkhovsky M.I. 2011. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta. 1807, 1398‒1413.
Forte E., Borisov V.B., Vicente J.B., Giuffre A. 2017. Cytochrome bd and gaseous ligands in bacterial physiology. Adv. Microb. Physiol. 71, 171‒234.
Borisov V.B., Siletsky S.A., Paiardini A., Hoogewijs D., Forte E., Giuffre A., Poole R.K. 2021. Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function and utility as drug targets. Antioxid. Redox Signal. 34, 1280‒1318.
Theßeling A., Rasmussen T., Burschel S., Wohlwend D., Kagi J., Muller R., Bottcher B., Friedrich T. 2019. Homologous bd oxidases share the same architecture but differ in mechanism. Nat. Commun. 10, 5138.
Safarian S., Hahn A., Mills D.J., Radloff M., Eisinger M.L., Nikolaev A., Meier-Credo J., Melin F., Miyoshi H., Gennis R.B., Sakamoto J., Langer J.D., Hellwig P., Kuhlbrandt W., Michel H. 2019. Active site rearrangement and structural divergence in prokaryotic respiratory oxidases. Science. 366, 100‒104.
Grauel A., Kagi J., Rasmussen T., Makarchuk I., Oppermann S., Moumbock A.F.A., Wohlwend D., Muller R., Melin F., Gunther S., Hellwig P., Bottcher B., Friedrich T. 2021. Structure of Escherichia coli cytochrome bd-II type oxidase with bound aurachin D. Nat. Commun. 12, 6498.
Grund T.N., Radloff M., Wu D., Goojani H.G., Witte L.F., Josting W., Buschmann S., Muller H., Elamri I., Welsch S., Schwalbe H., Michel H., Bald D., Safarian S. 2021. Mechanistic and structural diversity between cytochrome bd isoforms of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 118, e2114013118.
Friedrich T., Wohlwend D., Borisov V.B. 2022. Recent advances in structural studies of cytochrome bd and its potential application as a drug target. Int. J. Mol. Sci. 23, 3166.
Belevich I., Borisov V.B., Konstantinov A.A., Verkhovsky M.I. 2005. Oxygenated complex of cytochrome bd from Escherichia coli: Stability and photolability. FEBS Lett. 579, 4567‒4570.
Seaver L.C., Imlay J.A. 2004. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J. Biol. Chem. 279, 48742‒48750.
Mourenza A., Gil J.A., Mateos L.M., Letek M. 2020. Oxidative stress-generating antimicrobials, a novel strategy to overcome antibacterial resistance. Antioxidants (Basel). 9, 361.
Loewen P.C., Switala J., Triggs-Raine B.L. 1985. Catalases HPI and HPII in Escherichia coli are induced independently. Arch. Biochem. Biophys. 243, 144‒149.
Imlay J.A. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77, 755‒776.
Nobrega C.S., Devreese B., Pauleta S.R. 2018. YhjA—an Escherichia coli trihemic enzyme with quinol peroxidase activity. Biochim. Biophys. Acta Bioenerg. 1859, 411‒422.
Borisov V.B., Forte E., Davletshin A., Mastronicola D., Sarti P., Giuffre A. 2013. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: An additional defense against oxidative stress. FEBS Lett. 587, 2214‒2218.
Borisov V.B., Siletsky S.A., Nastasi M.R., Forte E. 2021. ROS defense systems and terminal oxidases in bacteria. Antioxidants (Basel). 10, 839.
Forte E., Nastasi M.R., Borisov V.B. 2022. Preparations of terminal oxidase cytochrome bd-II isolated from Escherichia coli reveal significant hydrogen peroxide scavenging activity. Biochemistry (Moscow). 87, 720‒730.
Deisseroth A., Dounce A.L. 1970. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol. Rev. 50, 319‒375.
Su S., Panmanee W., Wilson J.J., Mahtani H.K., Li Q., Vanderwielen B.D., Makris T.M., Rogers M., McDaniel C., Lipscomb J.D., Irvin R.T., Schurr M.J., Lancaster J.R., Jr., Kovall R.A., Hassett D.J. 2014. Catalase (KatA) plays a role in protection against anaerobic nitric oxide in Pseudomonas aeruginosa. PLoS One. 9, e91813.
Al-Attar S., Yu Y., Pinkse M., Hoeser J., Friedrich T., Bald D., de Vries S. 2016. Cytochrome bd displays significant quinol peroxidase activity. Sci. Rep. 6, 27631.
Goojani H.G., Besharati S., Chauhan P., Asseri A.H., Lill H., Bald D. 2023. Cytochrome bd-I from Escherichia coli is catalytically active in the absence of the CydH subunit. FEBS Lett. 597, 547‒556.
Forte E., Borisov V.B., Siletsky S.A., Petrosino M., Giuffre A. 2019. In the respiratory chain of Escherichia coli cytochromes bd-I and bd-II are more sensitive to carbon monoxide inhibition than cytochrome bo 3. Biochim. Biophys. Acta Bioenerg. 1860, 148088.
Borisov V.B. 2020. Effect of membrane environment on ligand-binding properties of the terminal oxidase cytochrome bd-I from Escherichia coli. Biochemistry (Moscow). 85, 1603‒1612.
Bloch D.A., Borisov V.B., Mogi T., Verkhovsky M.I. 2009. Heme/heme redox interaction and resolution of individual optical absorption spectra of the hemes in cytochrome bd from Escherichia coli. Biochim. Biophys. Acta. 1787, 1246–1253.
Borisov V.B., Davletshin A.I., Konstantinov A.A. 2010. Peroxidase activity of cytochrome bd from Escherichia coli. Biochemistry (Moscow). 75, 428‒436.
Edwards S.E., Loder C.S., Wu G., Corker H., Bainbridge B.W., Hill S., Poole R.K. 2000. Mutation of cytochrome bd quinol oxidase results in reduced stationary phase survival, iron deprivation, metal toxicity and oxidative stress in Azotobacter vinelandii. FEMS Microbiol. Lett. 185, 71‒77.
Endley S., McMurray D., Ficht T.A. 2001. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J. Bacteriol. 183, 2454‒2462.
Chang W., Small D.A., Toghrol F., Bentley W.E. 2006. Global transcriptome analysis of Staphylococcus aure-us response to hydrogen peroxide. J. Bacteriol. 188, 1648‒1659.
Small J.L., Park S.W., Kana B.D., Ioerger T.R., Sacchettini J.C., Ehrt S. 2013. Perturbation of cytochrome c maturation reveals adaptability of the respiratory chain in Mycobacterium tuberculosis. mBio. 4, e00475-13.
Forte E., Borisov V.B., Davletshin A., Mastronicola D., Sarti P., Giuffre A. 2013. Cytochrome bd oxidase and hydrogen peroxide resistance in Mycobacterium tuberculosis. mBio. 4, e01006‒e01013.
Lu P., Heineke M.H., Koul A., Andries K., Cook G.M., Lill H., van Spanning R., Bald D. 2015. The cytochrome bd-type quinol oxidase is important for survival of Mycobacterium smegmatis under peroxide and antibiotic-induced stress. Sci. Rep. 5, 10333.
Leclerc J., Rosenfeld E., Trainini M., Martin B., Meuric V., Bonnaure-Mallet M., Baysse C. 2015. The cytochrome bd oxidase of Porphyromonas gingivalis contributes to oxidative stress resistance and dioxygen tolerance. PLoS One. 10, e0143808.
Xia X., Wu S., Li L., Xu B., Wang G. 2018. The cytochrome bd complex is essential for chromate and sulfide resistance and is regulated by a GbsR-type regulator, CydE, in Alishewanella sp. WH16-1. Front. Microbiol. 9, 1849.
Wang P.-H., Sai W., Nie W.-H., Yan W., Iftikhar A., Ayizekeranmu Y., Huang J., Chen G.-y., Zhu B. 2022. A transferred regulator that contributes to Xanthomonas oryzae pv. Oryzicola oxidative stress adaptation and virulence by regulating the expression of cytochrome bd oxidase genes. J. Integr. Agric. 21, 1673‒1682.
Funding
This work was supported by the Russian Science Foundation (project no. 22-24-00045, https://rscf.ru/en/project/22-24-00045/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any research involving human participants or animals as research objects.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ADDITIONAL INFORMATION
The text was submitted by the author(s) in English.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borisov, V.B., Nastasi, M.R. & Forte, E. Cytochrome bd as Antioxidant Redox Enzyme. Mol Biol 57, 1077–1084 (2023). https://doi.org/10.1134/S0026893323060031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323060031