Abstract
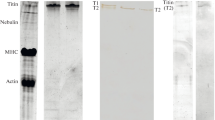
In this paper, the property of the muscle titin protein to form in vitro specific amyloid-like aggregates is discussed. The main difference from the known amyloid aggregates is the formation of a quaternary structure that resembles cross-β, with no changes in the secondary structure. Based on the results obtained earlier, as well as the results of this study, we make assumptions about changes in the structure of titin that occur during the formation of amyloid-like aggregates. In particular, our X-ray diffraction data on the titin aggregates suggest that β-strands in the aggregates of this protein are not located perpendicular to the fibril axis, as described for other amyloid proteins, but in parallel. The distance between the β-sheets in the aggregates may vary, and the β-sheets themselves are not strictly oriented along one of the axes, which can lead to the appearance of a diffuse ring reflection of ~8–12 Å. In this regard, the titin aggregates should not be called amyloid, but amyloid-like, with a quaternary structure that resembles cross-β. It cannot be excluded that the formation of this quaternary structure can also occur due to the partial unfolding of titin domains, followed by the interaction of open β-strands between neighboring domains and/or domains of neighboring molecules.



Similar content being viewed by others
REFERENCES
Tsytlonok M., Craig P.O., Sivertsson E., Serquera D., Perrett S., Best R.B., Wolynes P.G., Itzhaki L.S. 2013. Complex energy landscape of a giant repeat protein. Structure. 21 (11), 1954–1965. https://doi.org/10.1016/j.str.2013.08.028
Tian P., Best R.B. 2016. Best structural determinants of misfolding in multidomain proteins. PLOS Comput. Biol. 12 (5), e1004933. https://doi.org/10.1371/journal.pcbi.1004933
Apic G., Gough J., Teichmann S.A. 2001. Domain combinations in archaeal, eubacterial and eukaryotic proteomes. J. Mol. Biol. 310 (2), 311–325. https://doi.org/10.1006/jmbi.2001.4776
Ekman D., Björklund A.K., Frey-Skött J., Elofsson A. 2005. Multi-domain proteins in the three kingdoms of life: orphan domains and other unassigned regions. J. Mol. Biol. 348 (1), 231–243. https://doi.org/10.1016/j.jmb
Han J.H., Batey S., Nickson A.A., Teichmann S.A., Clarke J. 2007. The folding and evolution of multidomain proteins. J. Nat. Rev. Mol. Cell Biol. 8 (4), 319–330. https://doi.org/10.1038/nrm2144
Dobson C.M. 2003. Protein folding and misfolding. Nature.426 (6968), 884–890. https://doi.org/10.1038/nature02261
Rousseau F., Schymkowitz J., Itzhaki L.S. 2012. Implications of 3D domain swapping for protein folding, misfolding and function. Adv. Exp. Med. Biol. 747, 137–152. https://doi.org/10.1007/978-1-4614-3229-6_9
Knowles T.P., Vendruscolo M., Dobson C.M. 2014. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 15 (6), 384–396. https://doi.org/10.1038/nrm3810
Dobson C.M. 2004. Experimental investigation of protein folding and misfolding. Methods. 34 (1), 4–14. https://doi.org/10.1016/j.ymeth.2004.03.002
Buxbaum J.N., Linke R.P. 2000. A molecular history of the amyloidosis. J. Mol. Biol. 421 (2–3), 142–159. https://doi.org/10.1016/j.jmb.2012.01.024
Dobson C.M. 2004. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol.15 (1), 3–16. https://doi.org/10.1016/j.semcdb.2003.12.008
Freundt J.K., Linke W.A. 2019. Titin as a force-generating muscle protein under regulatory control. J. Appl. Physiol. 126 (5), 1474–1482. https://doi.org/10.1152/japplphysiol.00865.2018
Vikhlyantsev I.M., Podlubnaya Z.A. 2017. Nuances of electrophoresis study of titin/connectin. Biophys. Rev. 9 (3), 189–199. https://doi.org/10.1007/s12551-017-0266-6
Gregorio C.C., Granzier H., Sorimachi H., Labeit S. 1999. Muscle assembly: a titanic achievement? Curr. Opin. Cell Biol. 11 (1), 18–25. https://doi.org/10.1016/s0955-0674(99)80003-9
Giganti D., Yan K., Badilla C.L., Fernandez J.M., Alegre-Cebollada J. 2018. Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity. Nat. Commun. 9 (1), 185. https://doi.org/10.1038/s41467-017-02528-7
Bobylev A.G., Galzitskaya O.V., Fadeev R.S., Bobyleva L.G., Yurshenas D.A., Molochkov N.V., Dovidchenko N.V., Selivanova O.M., Penkov N.V., Podlubnaya Z.A., Vikhlyantsev I.M. 2016. Smooth muscle titin forms in vitro amyloid aggregates. Biosci. Repts.36 (3), pii: e00334. https://doi.org/10.1042/BSR20160066
Yakupova E.I., Vikhlyantsev I.M., Bobyleva L.G., Penkov N.V., Timchenko A.A., Timchenko M.A., Enin G.A., Khutzian S.S., Selivanova O.M., Bobylev A.G. 2018. Different amyloid aggregation of smooth muscles titin in vitro.J. Biomol. Struct. Dyn. 36 (9), 2237–2248. https://doi.org/10.1080/07391102.2017.1348988
Kim K., Keller T.C. 3rd. 2002. Smitin, a novel smooth muscle titin-like protein, interacts with myosin filaments in vivo and in vitro.J. Cell. Biol. 156 (1), 101–112. https://doi.org/10.1083/jcb.200107037
Fritz J.D., Swartz D.R., Greaser M.L. 1989. Factors affecting polyacryamide gel electrophoresis and electro-blotting of high-molecular-weight myofibrillar proteins. Anal. Biochem. 180 (2), 205–210. https://doi.org/10.1016/0003-2697(89)90116-4
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227 (5259), 680–685. https://doi.org/10.1038/227680a0
Towbin H., Staehelin T., Gordon J. 1989. Immunoblotting in the clinical laboratory. J. Clin. Chem. Clin. Biochem. 27 (8), 495–501.
Kumar S., Walter J. 2011. Phosphorylation of amyloid beta (Aβ) peptides: A trigger for formation of toxic aggregates in Alzheimer’s disease. Aging. 3 (8), 803–812 https://doi.org/10.18632/aging.100362
Sunde M., Serpell L.C., Bartlam M., Fraser P.E., Pepys M.B., Blake C.C. 1997. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 273 (3), 729–739. https://doi.org/10.1006/jmbi.1997.1348
Nelson R., Eisenberg D. 2006. Recent atomic models of amyloid fibril structure. Curr. Opin. Struct. Biol. 16 (2), 260–265. https://doi.org/10.1016/j.sbi.2006.03.007
Makin O.S., Serpell L.C. 2005. Structures for amyloid fibrils. FEBS J.272 (23), 5950–5961. https://doi.org/10.1111/j.1742-4658.2005.05025.x
Astbury W.T., Dickinson S., Bailey K. 1935. The X-ray diffraction interpretation of denaturation and the structure of seed globulins. Biochem. J.29 (10), 2351–2360. https://doi.org/10.1042/bj0292351
Eanes E.D., Glenner G.G. 1968. X-ray diffraction studies on amyloid filaments. J. Histochem. Cytochem. 16 (11), 673–677. https://doi.org/10.1177/16.11.673
Jahn T.R., Makin O.S., Morris K.L., Marshall K.E., Tian P., Sikorski P., Serpell L.C. 2010. The common architecture of cross-beta amyloid. J. Mol. Biol. 395 (4), 717–727. https://doi.org/10.1016/j.jmb.2009.09.039
Eisenberg D., Jucker M. 2012. The amyloid state of proteins in human diseases. Cell. 148 (6), 1188–1203. https://doi.org/10.1016/j.cell.2012.02.022
Wille H., Bian W., McDonald M., Kendall A., Colby D.W., Bloch L., Ollesch J., Borovinskiy A.L., Cohen F.E., Prusiner S.B., Stubbs G. 2009. Natural and synthetic prion structure from X-ray fiber diffraction. Proc. Natl. Acad. Sci. U. S. A.106 (40), 16990–16995. https://doi.org/10.1073/pnas.0909006106
Pauling L., Corey R. 1953. Two rippled-sheet configurations of polypeptide chains, and a note about the pleated sheets. Proc. Natl. Acad. Sci. U. S. A.39 (4), 253–256. https://doi.org/10.1073/pnas.39.4.253
Morris K.L., Serpell L.C. 2012. X-ray fibre diffraction studies of amyloid fibrils. Methods Mol. Biol. 849, 121–135. https://doi.org/10.1007/978-1-61779-551-0_9
Inouye H., Fraser P., Kirchner D. 1993. Structure of beta-crystallite assemblies formed by Alzheimer beta-amyloid protein analogues: analysis by X-ray diffraction. Biophys. J.64 (2), 502–519. https://doi.org/10.1016/S0006-3495(93)81393-6
Selivanova O.M., Grigorashvili E.I., Suvorina M.Y., Dzhus U.F., Nikulin A.D., Marchenkov V.V., Surin A.K., Galzitskaya O.V. 2016. X-ray diffraction and electron microscopy data for amyloid formation of Aβ40 and Aβ42. Data Brief. 8, 108–113. https://doi.org/10.1016/j.dib.2016.05.020
Clarke J., Cota E., Fowler S.B., Hamill S.J. 1999. Folding studies of immunoglobulin-like beta-sandwich proteins suggest that they share a common folding pathway. Structure. 7 (9), 1145–1153 https://doi.org/10.1016/s0969-2126(99)80181-6
Erickson H.P. 1994. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc. Natl. Acad. Sci. U. S. A.91 (21), 10114–10118. https://doi.org/10.1073/pnas.91.21.10114
Borgia A., Kemplen K.R., Borgia M.B., Soranno A., Shammas S., Wunderlich B., Nettels D., Best R.B., Clarke J., Schuler B. 2015. Transient misfolding dominates multidomain protein folding. Nat. Commun. 6, 8861. https://doi.org/10.1038/ncomms9861
Bianco P., Martonfalvi Z., Naftz K., Koszegi D., Kellermayer M. 2015. Titin domains progressively unfolded by force are homogenously distributed along the molecule. Biophys. J.109 (2), 340–345. https://doi.org/10.1016/j.bpj.2015.06.002
Martonfalvi Z., Bianco P., Naftz K., Ferenczy G.G., Kellermayer M. 2017. Force generation by titin folding. Protein Sci. 26 (7), 1380–1390. https://doi.org/10.1002/pro.3117
Rivas-Pardo J.A., Eckels E.C., Popa I., Kosuri P., Linke W.A., Fernández J.M. 2016. Work done by titin protein folding assists muscle contraction. Cell Reports. 14 (6), 1339–1347. https://doi.org/10.1016/j.celrep.2016.01.025
Eckels E.C., Haldar Sh., Tapia-Rojo R., Rivas-Pardo J.A., Fernández J.M. 2019. The mechanical power of titin folding. Cell Rep. 27 (6), 1836–1847. https://doi.org/10.1016/j.celrep.2019.04.046
Funding
This work was financially supported by the Russian Foundation for Basic Research (project nos. 18-04-00125, 19-34-90054 “Graduate students”) and a grant from the Russian Science Foundation (no. 19-74-10051) for E. Yakupova with use of the equipment of the Regional Pushchino Collective Use Center “Structural and Functional Research of Biosystems” of the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, and the Electron Microscopy Sector of the Central Scientific and Research Center of Biomedical Sciences, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare they have no conflict of interest.
This article does not contain any research involving humans or animals as research objects.
Rights and permissions
About this article
Cite this article
Bobylev, A.G., Yakupova, E.I., Bobyleva, L.G. et al. Changes in Titin Structure during Its Aggregation. Mol Biol 54, 578–585 (2020). https://doi.org/10.1134/S0026893320040044
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893320040044