Abstract
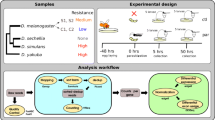
Drosophila melanogaster is the only invertebrate that contains endogenous retroviruses, which are called errantiviruses. Two domesticated genes, Grp and Iris, which originate from errantivirus gag and env, respectively, have been found in the D. melanogaster genome. The functions performed by the genes in Drosophila are still unclear. To identify the functions of domesticated gag and env in the D. melanogaster genome, expression of Iris and Grp was studied in strains differing by the presence or absence of the functional gypsy errantivirus. In addition, the expression levels were measured after injection of gram-positive and gram-negative bacteria, which activate different immune response pathways, and exposure to various abiotic stress factors. The presence of functional D. melanogaster retrovirus gypsy was found to increase the Grp expression level in somatic tissues of the carcass, while exerting no effect on the Iris expression level. Activation of the immune response in D. melanogaster by bacteria Bacillus cereus increased the Grp expression level and did not affect Iris expression. As for the effects of abiotic stress factors (oxidative stress, starvation, and heat and cold stress), the Grp expression level increased in response to starvation in D. melanogaster females, and the Iris expression level was downregulated in heat shock and oxidative stress. Based on the findings, Grp was assumed to play a direct role in the immune response in D. melanogaster; Iris is not involved in immune responses, but and apparently performs a cell function that is inhibited in stress.
Similar content being viewed by others
Abbreviations
- LTR:
-
long terminal repeat
- EPR:
-
endoplasmic reticulum
References
Miller W.J., McDonald J.F., Pinsker W. 1997. Molecular domestication of mobile elements. Genetica. 100, 261–270.
Jern P., Coffin J.M. 2008. Effects of retroviruses on host genome function. Annu Rev. Genet. 42, 709–732.
Pardue M.L., DeBaryshe P.G. 2003. Retrotransposons provide an evolutionarily robust non-telomerase mechanism to maintain telomeres. Annu Rev. Genet. 37, 485–511.
Brennecke J., Aravin A.A., Stark A., Dus M., Kellis M., Sachidanandam R., Hannon G.J. 2007. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 128, 1089–1103.
Lander E.S., Linton L.M., Birren B., Nusbaum C., et al. International Human Genome Sequencing Consortium. 2001. Initial sequencing and analysis of the human genome. Nature. 409, 860–921.
Volff J.N. 2006. Turning junk into gold: Domestication of transposable elements and the creation of new genes in eukaryotes. Bioessays. 28, 913–922.
Aravind L. 2000. Exploring histones and their relatives with the Histone Sequence Database. Trends Biochem. Sci. 25, 421–423.
Mi S., Lee X., Li X., Veldman G.M., Finnerty H., Racie L., La V.E., Tang X.Y., Edouard P., Howes S., Keith J.C.Jr., McCoy J.M. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 403, 785–789.
Stoye J.P., Coffin J.M. 2000. A provirus put to work. Nature. 403, 715–717.
Dupressoir A., Marceau G., Vernochet C., Bé nit L., Kanellopoulos C., Sapin V., Heidmann T. 2005. Syncytin- A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci. U. S. A. 102, 725–730.
Campillos M., Doerks T., Shah P.K., Bork P. 2006. Computational characterization of multiple Gag-like human proteins. Trends Genet. 22, 585–589.
Volff J.N. 2009. Cellular genes derived from Gypsy/Ty3 retrotransposons in mammalian genomes. Ann. NY Acad. Sci. 1178, 233–243.
Tan K.O., Tan K.M., Chan S.L., Yee K.S., Bevort M., Ang K.C., Yu V.C. 2001. MAP-1, a novel proapoptotic protein containing a BH3-like motif that associates with Bax through its Bcl-2 homology domains. J. Biol. Chem. 276, 2802–2807.
Yan Y., Buckler-White A., Wollenberg K., Kozak C.A. 2009. Origin, antiviral function and evidence for positive selection of the gammaretrovirus restriction gene Fv1 in the genus Mus. Proc. Natl. Acad. Sci. U. S. A. 106, 3259–3263.
Kim A., Terzian C., Santamaria P., Pé lisson A., Prud’ homme N., Bucheton A. 1994. Retroviruses in invertebrates: The gypsy retrotransposon is apparently an infectious retrovirus of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 91, 1285–1289.
Leblanc P., Desset S., Giorgi F., Taddei A.R., Fausto A.M., Mazzini M., Dastugue B., Vaury C. 2000. Life cycle of an endogenous retrovirus, ZAM, in Drosophila melanogaster. J. Virol. 74, 10658–10669.
Malik H.S., Henikoff S. 2005. Positive selection of Iris, a retroviral envelope-derived host gene in Drosophila melanogaster. PLoS Genet. 1, e44.
Nefedova L., Kuzmin I., Makhnovskii P., Kim A. 2014. Domesticated retroviral GAG gene in Drosophila: New functions for an old gene. Virology. 450–451, 196–204. doi 10.1016/jvirol.2013.12.024
Kim A.I., Belyaeva E.S., Larkina Z.G., Aslanyan M.M. 1989. Genetic instability and MDG4 mobile element transposition in a Drosophila melanogaster mutator line. Genetika (Moscow). 25, 1747–1756.
Jordan K.W., Craver K.L., Magwire M.M., Cubilla C.E., Mackay T.F., Anholt R.R. 2012. Genome-wide association for sensitivity to chronic oxidative stress in Drosophila melanogaster. PLoS ONE. 7, e38722.
Kingsolver M.B., Huang Z., Hardy R.W. 2013. Insect antiviral innate immunity: Pathways, effectors, and connections. J. Mol. Biol. 425, 4921–4936.
Nefedova L.N., Kuz’ min I.V., Burmistrova D.A., Rezazadeh C., Kim A.I. 2011. Transcriptional analysis of the Grp gene, a genomic homolog of the retrotransposon gypsy gag gene, in Drosophila melanogaster. Russ. J. Genet. 47 (8), 912–916.
Aggarwal K., Silverman N. 2008. Positive and negative regulation of the Drosophila immune response. BMB Rep. 41, 267–277.
Dostert C., Jouanguy E., Irving P., Troxler L., Galiana- Arnoux D., Hetru C., Hoffmann J. Imler J.-L. 2005. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 6, 946–953.
Davies S.A., Overend G., Sebastian S., Cundall M., Cabrero P., Dow J.A, Terhzaz S. 2012. Immune and stress response ‘cross-talk’ in the Drosophila Malpighian tubule. J. Insect Physiol. 58, 488–497.
Huang Z., Kingsolver M.B., Avadhanula V., Hardy R.W. 2013. An antiviral role for antimicrobial peptides during the arthropod response to alphavirus replication. J. Virology. 87 (8), 4272–4280.
Guruharsha K.G., Rual J.F., Zhai B., Mintseris J., Vaidya P., Vaidya N., Beekman C., Wong C., Rhee D.Y., Cenaj O., McKillip E., Shah S., Stapleton M., Wan K.H., Yu C., et al. 2011. A protein complex network of Drosophila melanogaster. Cell. 147, 690–703.
Imaoka S. 2011. Chemical stress on protein disulfide isomerases and inhibition of their functions. Int. Rev. Cell Mol. Biol. 290, 121–166.
Nielsen M.D., Luo X., Biteau B., Syverson K., Jasper H. 2008. 14-3-3 Epsilon antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell. 7, 688–699.
Majzoub K., Hafirassou M.L., Meignin C., Goto A., Marzi S., Fedorova A., Verdier Y., Vinh J., Hoffmann J.A., Martin F., Baumert T.F., Schuster C., Imler J.L. 2014. RACK1 controls IRES-mediated translation of viruses. Cell. 159, 1086–1095.
Hanson P.J., Zhang H.M., Hemida M.G., Ye X., Qiu Y., Yang D. 2012. IRES-dependent translational control during virus-induced endoplasmic reticulum stress and apoptosis. Front. Microbiol. 3, 92.
Pisarev A.V., Shirokikh N.E., Hellen C.U. 2005. Translation initiation by factor-independent binding of eukaryotic ribosomes to internal ribosomal entry sites. C. R. Biol. 328, 589–605.
Ronfort C., de Breyne S., Sandrin V., Darlix J.L., Ohlmann T. 2004. Characterization of two distinct RNA domains that regulate translation of the Drosophila gypsy retroelement. RNA. 10, 504–515.
Wang L., Ryoo H.D., Qi Y., Jasper H. 2015. PERK limits Drosophila lifespan by promoting intestinal stem cell proliferation in response to ER stress. PLoS Genet. 11, e1005220.
van der Kant R., Fish A., Janssen L., Janssen H., Krom S., Ho N., Brummelkamp T., Carette J., Rocha N., Neefjes J. 2013. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J. Cell Sci. 126, 3462–3474.
Baril C., Sahmi M., Ashton-Beaucage D., Stronach B., Therrien M. 2009. The PP2C Alphabet is a negative regulator of stress-activated protein kinase signaling in Drosophila. Genetics. 181, 567–579.
Rybakin V., Clemen C.S. 2005. Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking. Bioessays. 27, 625–632.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © P.A. Makhnovskii, I.V. Kuzmin, L.N. Nefedova, A.I. Kim, 2016, published in Molekulyarnaya Biologiya, 2016, Vol. 50, No. 3, pp. 435–444.
Rights and permissions
About this article
Cite this article
Makhnovskii, P.A., Kuzmin, I.V., Nefedova, L.N. et al. Functional analysis of Grp and Iris, the gag and env domesticated errantivirus genes, in the Drosophila melanogaster genome. Mol Biol 50, 379–386 (2016). https://doi.org/10.1134/S0026893316020151
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893316020151