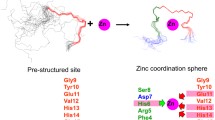
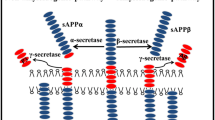
Abstract
Aggregation of the β-amyloid peptide (Aβ) underlies the development of Alzheimer’s disease. The review considers the main steps of the Aβ formation and aggregation. Emphasis is placed on the interaction of zinc ions with the metal-binding domain 1–16 of Aβ as a molecular mechanism leading to Aβ aggregation. Recent studies of native modifications in the Aβ metal-binding domain revealed its structural polymorphism. The prospects of further studying the modifications to determine the pathogenetic mechanism of Aβ aggregation are discussed.
Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
β-amyloid
- APP:
-
amyloid precursor protein
- CSF:
-
cerebrospinal fluid
References
Shelkovnikova T.A., Kulikova A.A., Tsvetkov Ph.O., Peters O., Bachurin S.O., Buchman V.L., Ninkina N.N. 2012. Proteinopathies, neurodegenerative disorders with protein aggregation-based pathology. Mol. Biol. (Moscow). 46, 362–374.
Hof P.R., Bouras C., Perl D.P., Sparks D.L., Mehta N., Morrison J.H. 1995. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down’s syndrome. Quantitative regional analysis and comparison with Alzheimer’s disease. Arch. Neurol. 52, 379–391.
Gavrilova S.I. 2007. Farmakoterapiya bolezni Al’tsgeimera (Pharmacological Treatment of Alzheimer’s Disease). Moscow: Pul’s.
Selkoe D.J. 2001. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 81, 741–766.
Price D.L., Borchelt D.R., Sisodia S.S. 1993. Alzheimer disease and the prion disorders amyloid beta-protein and prion protein amyloidoses. Proc. Natl. Acad. Sci. U. S. A. 90, 6381–6384.
Morris J.C., Storandt M., McKeel D.W., Jr., Rubin E.H., Price J.L., Grant E.A., Berg L. 1996. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 46, 707–719.
Gavrilova S.I., Zharikov G.A. 2001. Modern strategies in pathogenetic treatment of Alzheimer’s disease. Vestn. Ross. Akad. Med. Nauk. 7, 13–18.
Hy L.X., Keller D.M. 2000. Prevalence of AD among whites: a summary by levels of severity. Neurology. 55, 198–204.
Wortmann M. 2012. Dementia: A global health priority. Highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 4, 40.
Duyckaerts C., Delatour B., Potier M.C. 2009. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 118, 5–36.
Holtzman D.M., Morris J.C., Goate A.M. 2011. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 3, 77sr1.
Selkoe D.J. 2008. Biochemistry and molecular biology of amyloid beta-protein and the mechanism of Alzheimer’s disease. Handbook Clin. Neurol. 89, 245–260.
LaFerla F.M. 2010. Pathways linking Abeta and tau pathologies. Biochem. Soc. Trans. 38, 993–995.
Hardy J., Selkoe D.J. 2002. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 297, 353–356.
Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A., Regan C.M., Walsh D.M., Sabatini B.L., Selkoe D.J. 2008. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842.
Haass C. 2010. Initiation and propagation of neurodegeneration. Nat. Med. 16, 1201–1204.
Haass C., Selkoe D.J. 2007. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112.
Lacor P.N., Buniel M.C., Chang L., Fernandez S.J., Gong Y., Viola K.L., Lambert M.P., Velasco P.T., Bigio E.H., Finch C.E., Krafft G.A., Klein W.L. 2004. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J. Neurosci. 24, 10191–10200.
Lacor P.N., Buniel M.C., Furlow P.W., Clemente A.S., Velasco P.T., Wood M., Viola K.L., Klein W.L. 2007. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 27, 796–807.
Choi S.H., Bosetti F. 2009. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging (Albany, NY). 1, 234–244.
Candelario-Jalil E. 2009. A role for cyclooxygenase-1 in beta-amyloid-induced neuroinflammation. Aging (Albany, NY). 1, 350–353.
Pratico D., Trojanowski J.Q. 2000. Inflammatory hypotheses: Novel mechanisms of Alzheimer’s neurodegeneration and new therapeutic targets?. Neurobiol. Aging. 21, 441–445, 451–443.
Massaad C.A., Pautler R.G., Klann E. 2009. Mitochondrial superoxide: A key player in Alzheimer’s disease. Aging (Albany, NY). 1, 758–761.
Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., Wals P., Zhang C., Finch C.E., Krafft G.A., Klein W.L. 1998. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 95, 6448–6453.
Jin M., Shepardson N., Yang T., Chen G., Walsh D., Selkoe D.J. 2011. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. U. S. A. 108, 5819–5824.
Treusch S., Cyr D.M., Lindquist S. 2009. Amyloid deposits: Protection against toxic protein species?. Cell Cycle. 8, 1668–1674.
Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C., et al. 1992. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 359, 325–327.
Haass C., Schlossmacher M.G., Hung A.Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B.L., Lieberburg I., Koo E.H., Schenk D., Teplow D.B., et al. 1992. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 359, 322–325.
Kang J., Lemaire H.G., Unterbeck A., Salbaum J.M., Masters C.L., Grzeschik K.H., Multhaup G., Beyreuther K., Muller-Hill B. 1987. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 325, 733–736.
Anliker B., Muller U. 2006. The functions of mammalian amyloid precursor protein and related amyloid precursor-like proteins. Neurodegener. Dis. 3, 239–246.
Walsh D.M., Minogue A.M., Sala Frigerio C., Fadeeva J.V., Wasco W., Selkoe D.J. 2007. The APP family of proteins: similarities and differences. Biochem. Soc. Trans. 35, 416–420.
Kerrigan T.L., Atkinson L., Peers C., Pearson H.A. 2008. Modulation of’ A’-type K+ current by rodent and human forms of amyloid beta protein. Neuroreports. 19, 839–843.
Tabaton M., Zhu X., Perry G., Smith M.A., Giliberto L. 2010. Signaling effect of amyloid-beta(42) on the processing of AbetaPP. Exp. Neurol. 221, 18–25.
Yao Z.X., Papadopoulos V. 2002. Function of betaamyloid in cholesterol transport: A lead to neurotoxicity. FASEB J. 16, 1677–1679.
Zou K., Gong J.S., Yanagisawa K., Michikawa M. 2002. A novel function of monomeric amyloid betaprotein serving as an antioxidant molecule against metal-induced oxidative damage. J. Neurosci. 22, 4833–4841.
Morley J.E., Farr S.A., Banks W.A., Johnson S.N., Yamada K.A., Xu L. 2010. A physiological role for amyloid-beta protein:enhancement of learning and memory. J. Alzheimer’s Dis. 19, 441–449.
Bailey J.A., Maloney B., Ge Y.W., Lahiri D.K. 2011. Functional activity of the novel Alzheimer’s amyloid beta-peptide interacting domain (AbetaID) in the APP and BACE1 promoter sequences and implications in activating apoptotic genes and in amyloidogenesis. Gene. 488, 13–22.
Soscia S.J., Kirby J.E., Washicosky K.J., Tucker S.M., Ingelsson M., Hyman B., Burton M.A., Goldstein L.E., Duong S., Tanzi R.E., Moir R.D. 2010. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS ONE. 5, e9505.
Kojro E., Fahrenholz F. 2005. The non-amyloidogenic pathway: Structure and function of alpha-secretases. Subcell. Biochem. 38, 105–127.
Pastor P., Goate A.M. 2004. Molecular genetics of Alzheimer’s disease. Curr. Psychiatry Rep. 6, 125–133.
Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A.Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D.J. 1992. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 360, 672–674.
Herl L., Thomas A.V., Lill C.M., Banks M., Deng A., Jones P.B., Spoelgen R., Hyman B.T., Berezovska O. 2009. Mutations in amyloid precursor protein affect its interactions with presenilin/gamma-secretase. Mol. Cell Neurosci. 41, 166–174.
Haass C., Hung A.Y., Selkoe D.J., Teplow D.B. 1994. Mutations associated with a locus for familial Alzheimer’s disease result in alternative processing of amyloid beta-protein precursor. J. Biol. Chem. 269, 17741–17748.
Chiti F., Stefani M., Taddei N., Ramponi G., Dobson C.M. 2003. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 424, 805–808.
Nadezhdin K.D., Bocharova O.V., Bocharov E.V., Arseniev A.S. 2011. Structural and dynamic study of the transmembrane domain of the amyloid precursor protein. Acta Naturae. 3, 69–76.
Nadezhdin K.D., Bocharova O.V., Bocharov E.V., Arseniev A.S. 2012. Dimeric structure of transmembrane domain of amyloid precursor protein in micellar environment. FEBS Lett. 586, 1687–1692.
De Strooper B. 2007. Loss-of-function presenilin mutations in Alzheimer disease: Talking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 8, 141–146.
Yoshiike Y., Minai R., Matsuo Y., Chen Y.R., Kimura T., Takashima A. 2008. Amyloid oligomer conformation in a group of natively folded proteins. PLoS ONE. 3, e3235.
Prusiner S.B. 2012. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 336, 1511–1513.
Ni C.L., Shi H.P., Yu H.M., Chang Y.C., Chen Y.R. 2011. Folding stability of amyloid-beta 40 monomer is an important determinant of the nucleation kinetics in fibrillization. FASEB J. 25, 1390–1401.
Harper J.D., Lansbury P.T., Jr. 1997. Models of amyloid seeding in Alzheimer’s disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66, 385–407.
Naiki H., Gejyo F. 1999. Kinetic analysis of amyloid fibril formation. Methods Enzymol. 309, 305–318.
Kumar S., Walter J. 2011. Phosphorylation of amyloid beta (Abeta) peptides: A trigger for formation of toxic aggregates in Alzheimer’s disease. Aging (Albany, NY). 3, 803–812.
Roychaudhuri R., Yang M., Hoshi M.M., Teplow D.B. 2009. Amyloid beta protein assembly and Alzheimer disease. J. Biol. Chem. 284, 4749–4753.
Du D., Murray A.N., Cohen E., Kim H.E., Simkovsky R., Dillin A., Kelly J.W. 2011. A kinetic aggregation assay allowing selective and sensitive amyloid-beta quantification in cells and tissues. Biochemistry. 50, 1607–1617.
Kane M.D., Lipinski W.J., Callahan M.J., Bian F., Durham R.A., Schwarz R.D., Roher A.E., Walker L.C. 2000. Evidence for seeding of beta-amyloid by intracerebral infusion of Alzheimer brain extracts in betaamyloid precursor protein-transgenic mice. J. Neurosci. 20, 3606–3611.
Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A.L., Vigouret J.M., Paganetti P., Walsh D.M., Mathews P.M., Ghiso J., Staufenbiel M., Walker L.C., Jucker M. 2006. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 313, 1781–1784.
Eisele Y.S., Bolmont T., Heikenwalder M., Langer F., Jacobson L.H., Yan Z.X., Roth K., Aguzzi A., Staufenbiel M., Walker L.C., Jucker M. 2009. Induction of cerebral beta-amyloidosis: Intracerebral versus systemic Abeta inoculation. Proc. Natl. Acad. Sci. U. S. A. 106, 12926–12931.
Watts J.C., Giles K., Grillo S.K., Lemus A., DeArmond S.J., Prusiner S.B. 2011. Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 108, 2528–2533.
Jucker M., Walker L.C. 2011. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 70, 532–540.
Eisele Y.S., Obermuller U., Heilbronner G., Baumann F., Kaeser S.A., Wolburg H., Walker L.C., Staufenbiel M., Heikenwalder M., Jucker M. 2010. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 330, 980–982.
Langer F., Eisele Y.S., Fritschi S.K., Staufenbiel M., Walker L.C., Jucker M. 2011. Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J. Neurosci. 31, 14488–14495.
Petkova A.T., Leapman R.D., Guo Z., Yau W.M., Mattson M.P., Tycko R. 2005. Self-propagating, molecular-level polymorphism in Alzheimer’s betaamyloid fibrils. Science. 307, 262–265.
Nilsson K.P., Aslund A., Berg I., Nystrom S., Konradsson P., Herland A., Inganas O., Stabo-Eeg F., Lindgren M., Westermark G.T., Lannfelt L., Nilsson L.N., Hammarstrom P. 2007. Imaging distinct conformational states of amyloid-beta fibrils in Alzheimer’s disease using novel luminescent probes. ACS Chem. Biol. 2, 553–560.
Levine H., 3rd, Walker L.C. 2010. Molecular polymorphism of Abeta in Alzheimer’s disease. Neurobiol. Aging. 31, 542–548.
Stohr J., Watts J.C., Mensinger Z.L., Oehler A., Grillo S.K., DeArmond S.J., Prusiner S.B., Giles K. 2012. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc. Natl. Acad. Sci. U. S. A. 109, 11025–11030.
Aguzzi A., Rajendran L. 2009. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 64, 783–790.
Colby D.W., Prusiner S.B. 2011. Prions. Cold Spring Harbor Perspect. Biol. 3, a006833.
Wadsworth J.D., Collinge J. 2011. Molecular pathology of human prion disease. Acta Neuropathol. 121, 69–77.
Weissmann C. 2004. The state of the prion. Nat. Rev. Microbiol. 2, 861–871.
Aguzzi A. 2007. Prion biology: The quest for the test. Nat. Methods. 4, 614–616.
Collinge J., Clarke A.R. 2007. A general model of prion strains and their pathogenicity. Science. 318, 930–936.
Pike C.J., Walencewicz A.J., Glabe C.G., Cotman C.W. 1991. Aggregation-related toxicity of synthetic betaamyloid protein in hippocampal cultures. Eur. J. Pharmacol. 207, 367–368.
Lorenzo A., Yankner B.A. 1994. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. U. S. A. 91, 12243–12247.
Hartley D.M., Walsh D.M., Ye C.P., Diehl T., Vasquez S., Vassilev P.M., Teplow D.B., Selkoe D.J. 1999. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876–8884.
Townsend M., Shankar G.M., Mehta T., Walsh D.M., Selkoe D.J. 2006. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: A potent role for trimers. J. Physiol. 572, 477–492.
Selkoe D.J. 2008. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 192, 106–113.
Shankar G.M., Bloodgood B.L., Townsend M., Walsh D.M., Selkoe D.J., Sabatini B.L. 2007. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875.
Li S., Hong S., Shepardson N.E., Walsh D.M., Shankar G.M., Selkoe D. 2009. Soluble oligomers of amyloid beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 62, 788–801.
Cleary J.P., Walsh D.M., Hofmeister J.J., Shankar G.M., Kuskowski M.A., Selkoe D.J., Ashe K.H. 2005. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 8, 79–84.
Walsh D.M., Selkoe D.J. 2007. A beta oligomers: A decade of discovery. J. Neurochem. 101, 1172–1184.
Klein W.L., Krafft G.A., Finch C.E. 2001. Targeting small Abeta oligomers: The solution to an Alzheimer’s disease conundrum?. Trends Neurosci. 24, 219–224.
Lublin A.L., Gandy S. 2010. Amyloid-beta oligomers: Possible roles as key neurotoxins in Alzheimer’s disease. Mt. Sinai J. Med. 77, 43–49.
Cappai R., Barnham K.J. 2008. Delineating the mechanism of Alzheimer’s disease A beta peptide neurotoxicity. Neurochem. Res. 33, 526–532.
Simakova O., Arispe N.J. 2007. The cell-selective neurotoxicity of the Alzheimer’s Abeta peptide is determined by surface phosphatidylserine and cytosolic ATP levels: Membrane binding is required for Abeta toxicity. J. Neurosci. 27, 13719–13729.
Mitkevich V.A., Petrushanko I.Y., Yegorov Y.E., Simonenko O.V., Vishnyakova K.S., Kulikova A.A., Tsvetkov P.O., Makarov A.A., Kozin S.A. 2013. Isomerization of Asp7 leads to increased toxic effect of amyloidbeta42 on human neuronal cells. Cell Death Dis. 4, e939.
Atwood C.S., Martins R.N., Smith M.A., Perry G. 2002. Senile plaque composition and posttranslational modification of amyloid-beta peptide and associated proteins. Peptides. 23, 1343–1350.
Walker L.C., LeVine H. 2000. The cerebral proteopathies: Neurodegenerative disorders of protein conformation and assembly. Mol. Neurobiol. 21, 83–95.
Finder V.H., Glockshuber R. 2007. Amyloid-beta aggregation. Neurodegener. Dis. 4, 13–27.
Crouch P.J., Cimdins K., Duce J.A., Bush A.I., Trounce I.A. 2007. Mitochondria in aging and Alzheimer’s disease. Rejuvenation Res. 10, 349–357.
Mawuenyega K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C., Yarasheski K.E., Bateman R.J. 2010. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 330, 1774.
Iwata N., Higuchi M., Saido T.C. 2005. Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol. Ther. 108, 129–148.
Bush A.I. 2013. The metal theory of Alzheimer’s disease. J. Alzheimers Dis. 33 Suppl. 1, S277–S281.
Frederickson C.J., Bush A.I. 2001. Synaptically released zinc: Physiological functions and pathological effects. Biometals. 14, 353–366.
Hooper J. 1996. Mental disorders: Targeting the brain. Time. 148, 46–50.
Frederickson C.J., Danscher G. 1990. Zinc-containing neurons in hippocampus and related CNS structures. Prog. Brain Res. 83, 71–84.
Slomianka L., Danscher G., Frederickson C.J. 1990. Labeling of the neurons of origin of zinc-containing pathways by intraperitoneal injections of sodium selenite. Neuroscience. 38, 843–854.
Koh J.Y. 2005. Endogenous zinc in neurological diseases. J. Clin. Neurol. 1, 121–133.
Paoletti P., Vergnano A.M., Barbour B., Casado M. 2009. Zinc at glutamatergic synapses. Neuroscience. 158, 126–136.
Edbauer D., Winkler E., Regula J.T., Pesold B., Steiner H., Haass C. 2003. Reconstitution of gammasecretase activity. Nat. Cell Biol. 5, 486–488.
Edwards D.R., Handsley M.M., Pennington C.J. 2008. The ADAM metalloproteinases. Mol. Aspects Med. 29, 258–289.
Leissring M.A. 2008. The AbetaCs of Abeta-cleaving proteases. J. Biol. Chem. 283, 29645–29649.
Khmeleva S.A., Mezentsev Y.V., Kozin S.A., Tsvetkov P.O., Ivanov A.S., Bodoev N.V., Makarov A.A., Radko S.P. 2013. Zinc-induced interaction of the metal-binding domain of amyloid-beta peptide with DNA. J. Alzheimer’s Dis. 36, 633–636.
Lovell M.A., Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. 1998. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 158, 47–52.
Lee J.Y., Cole T.B., Palmiter R.D., Suh S.W., Koh J.Y. 2002. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 99, 7705–7710.
Miller L.M., Wang Q., Telivala T.P., Smith R.J., Lanzirotti A., Miklossy J. 2006. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 155, 30–37.
Dong J., Atwood C.S., Anderson V.E., Siedlak S.L., Smith M.A., Perry G., Carey P.R. 2003. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 42, 2768–2773.
Bush A.I., Pettingell W.H., Multhaup G., de Paradis M., Vonsattel J.P., Gusella J.F., Beyreuther K., Masters C.L., Tanzi R.E. 1994. Rapid induction of Alzheimer Abeta amyloid formation by zinc. Science. 265, 1464–1467.
Lim K.H., Kim Y.K., Chang Y.T. 2007. Investigations of the molecular mechanism of metal-induced Abeta(1–40) amyloidogenesis. Biochemistry. 46, 13523–13532.
Deshpande A., Kawai H., Metherate R., Glabe C.G., Busciglio J. 2009. A role for synaptic zinc in activitydependent Abeta oligomer formation and accumulation at excitatory synapses. J. Neurosci. 29, 4004–4015.
Miller Y., Ma B., Nussinov R. 2010. Zinc ions promote Alzheimer Abeta aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. U. S. A. 107, 9490–9495.
Kozin S.A., Zirah S., Rebuffat S., Hoa G.H., Debey P. 2001. Zinc binding to Alzheimer’s Abeta(1-16) peptide results in stable soluble complex. Biochem. Biophys. Res. Commun. 285, 959–964.
Mekmouche Y., Coppel Y., Hochgrafe K., Guilloreau L., Talmard C., Mazarguil H., Faller P. 2005. Characterization of the ZnII binding to the peptide amyloidbeta1-16 linked to Alzheimer’s disease. ChemBioChem. 6, 1663–1671.
Syme C.D., Viles J.H. 2006. Solution 1H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Abeta) of Alzheimer’s disease. Biochim. Biophys. Acta. 1764, 246–256.
Talmard C., Bouzan A., Faller P. 2007. Zinc binding to amyloid-beta: Isothermal titration calorimetry and Zn competition experiments with Zn sensors. Biochemistry. 46, 13658–13666.
Zirah S., Kozin S.A., Mazur A.K., Blond A., Cheminant M., Segalas-Milazzo I., Debey P., Rebuffat S. 2006. Structural changes of region 1-16 of the Alzheimer disease amyloid beta-peptide upon zinc binding and in vitro aging. J. Biol. Chem. 281, 2151–2161.
Faller P., Hureau C. 2009. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans. 7, 1080–1094.
Danielsson J., Pierattelli R., Banci L., Graslund A. 2007. High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid beta-peptide. FEBS J. 274, 46–59.
Zirah S., Rebuffat S., Kozin S.A., Debey P., Fournier F., Lesage D., Tabet J.C. 2003. Zinc binding properties of the amyloid fragment A beta(1-16) studied by electrospray-ionization mass spectrometry. Int. J. Mass Spectrometry. 228, 999–1016.
Curtain C.C., Ali F., Volitakis I., Cherny R.A., Norton R.S., Beyreuther K., Barrow C.J., Masters C.L., Bush A.I., Barnham K.J. 2001. Alzheimer’s disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J. Biol. Chem. 276, 20466–20473.
Liu S.T., Howlett G., Barrow C.J. 1999. Histidine-13 is a crucial residue in the zinc ion-induced aggregation of the A beta peptide of Alzheimer’s disease. Biochemistry. 38, 9373–9378.
Yang D.S., McLaurin J., Qin K., Westaway D., Fraser P.E. 2000. Examining the zinc binding site of the amyloid-beta peptide. Eur. J. Biochem. 267, 6692–6698.
Gaggelli E., Grzonka Z., Kozlowski H., Migliorini C., Molteni E., Valensin D., Valensin G. 2008. Structural features of the Cu(II) complex with the rat Abeta(1-28) fragment. Chem. Commun. (Cambridge). 3, 341–343.
Tsvetkov P.O., Kulikova A.A., Golovin A.V., Tkachev Y.V., Archakov A.I., Kozin S.A., Makarov A.A. 2010. Minimal Zn2+ binding site of amyloid-beta. Biophys. J. 99, L84–L86.
Kozin S.A., Tsvetkov P.O., Kulikova A.A., Indeikina M.I., Mezentsev Y.V., Istrate A.N. Popov I.A., Ivanov A.S., Polshakov V.I., Makarov A.A. 2014. Zinc-induced dimers of chemically modified Aβ are possible aggregation seeds. Alzheimer’s Dementia. 10, 793.
O’Malley T.T., Oktaviani N.A., Zhang D., Lomakin A., O’Nuallain B., Linse S., Benedek G.B., Rowan M.J., Mulder F.A., Walsh D.M. 2014. Abeta dimers differ from monomers in structural propensity, aggregation paths and population of synaptotoxic assemblies. Biochem. J. 461, 413–426.
Kozin S.A., Mezentsev Y.V., Kulikova A.A., Indeykina M.I., Golovin A.V., Ivanov A.S., Tsvetkov P.O., Makarov A.A. 2011. Zinc-induced dimerization of the amyloid-beta metal-binding domain 1-16 is mediated by residues 11-14. Mol. Biosyst. 7, 1053–1055.
Portelius E., Zetterberg H., Andreasson U., Brinkmalm G., Andreasen N., Wallin A., Westman-Brinkmalm A., Blennow K. 2006. An Alzheimer’s diseasespecific beta-amyloid fragment signature in cerebrospinal fluid. Neurosci. Lett. 409, 215–219.
Istrate A.N., Tsvetkov P.O., Mantsyzov A.B., Kulikova A.A., Kozin S.A., Makarov A.A., Polshakov V.I. 2012. NMR solution structure of rat abeta(1-16): Toward understanding the mechanism of rats’ resistance to Alzheimer’s disease. Biophys. J. 102, 136–143.
Istrate A.N., Mantsyzov A.B., Kozin S.A., Polshakov V.I. 2010. Optimization of the methods for small peptide solution structure determination by NMR spectroscopy. Mol. Biol. (Moscow). 44, 958–967.
Di Fede G., Catania M., Morbin M., Rossi G., Suardi S., Mazzoleni G., Merlin M., Giovagnoli A.R., Prioni S., Erbetta A., Falcone C., Gobbi M., Colombo L., Bastone A., Beeg M., Manzoni C., Francescucci B., Spagnoli A., Cantu L., Del Favero E., Levy E., Salmona M., Tagliavini F. 2009. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 323, 1473–1477.
Giaccone G., Morbin M., Moda F., Botta M., Mazzoleni G., Uggetti A., Catania M., Moro M.L., Redaelli V., Spagnoli A., Rossi R.S., Salmona M., Di Fede G., Tagliavini F. 2010. Neuropathology of the recessive A673V APP mutation: Alzheimer disease with distinctive features. Acta Neuropathol. 120, 803–812.
Diomede L., Di Fede G., Romeo M., Bagnati R., Ghidoni R., Fiordaliso F., Salio M., Rossi A., Catania M., Paterlini A., Benussi L., Bastone A., Stravalaci M., Gobbi M., Tagliavini F., Salmona M. 2014. Expression of A2V-mutated Abeta in Caenorhabditis elegans results in oligomer formation and toxicity. Neurobiol. Dis. 62, 521–532.
Ono K., Condron M.M., Teplow D.B. 2010. Effects of the English (H6R) and Tottori (D7N) familial Alzheimer disease mutations on amyloid beta-protein assembly and toxicity. J. Biol. Chem. 285, 23186–23197.
Hori Y., Hashimoto T., Wakutani Y., Urakami K., Nakashima K., Condron M.M., Tsubuki S., Saido T.C., Teplow D.B., Iwatsubo T. 2007. The Tottori (D7N) and English (H6R) familial Alzheimer disease mutations accelerate Abeta fibril formation without increasing protofibril formation. J. Biol. Chem. 282, 4916–4923.
Chen W.T., Hong C.J., Lin Y.T., Chang W.H., Huang H.T., Liao J.Y., Chang Y.J., Hsieh Y.F., Cheng C.Y., Liu H.C., Chen Y.R., Cheng I.H. 2012. Amyloid-beta (Abeta) D7H mutation increases oligomeric Abeta42 and alters properties of Abeta-zinc/copper assemblies. PLoS ONE. 7, e35807.
Xu L., Chen Y., Wang X. 2014. Dual effects of familial Alzheimer’s disease mutations (D7H, D7N, and H6R) on amyloid beta peptide: Correlation dynamics and zinc binding. Proteins. 82(12), 3286–3297. doi 10.1002/prot.24669
Kaden D., Harmeier A., Weise C., Munter L.M., Althoff V., Rost B.R., Hildebrand P.W., Schmitz D., Schaefer M., Lurz R., Skodda S., Yamamoto R., Arlt S., Finckh U., Multhaup G. 2012. Novel APP/Abeta mutation K16N produces highly toxic heteromeric Abeta oligomers. EMBO Mol. Med. 4, 647–659.
Popov I.A., Indeikina M.I., Kononikhin A.S., Starodubtseva N.L., Kozin S.A., Makarov A.A., Nikolaev E.N. 2013. ESI-MS identification of the minimal zincbinding center in natural isoforms of β-amyloid domain 1–16. Mol. Biol. (Moscow). 47, 440–445.
Kozin S.A., Kulikova A.A., Istrate A.N., Tsvetkov P.O., Zhokhov S.S., Mezentsev Y.V., Ivanov A.S., Polshakov V.I., Makarov A.A. 2015. The English (H6R) familial Alzheimer’s disease mutation facilitates zincinduced dimerization of the amyloid-β metal-binding domain. Metallomics. DOI: 10.1039/C4MT00259H.
Saido T.C., Yamao-Harigaya W., Iwatsubo T., Kawashima S. 1996. Amino- and carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neurosci. Lett. 215, 173–176.
Roher A.E., Lowenson J.D., Clarke S., Woods A.S., Cotter R.J., Gowing E., Ball M.J. 1993. beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 90, 10836–10840.
Roher A.E., Palmer K.C., Yurewicz E.C., Ball M.J., Greenberg B.D. 1993. Morphological and biochemical analyses of amyloid plaque core proteins purified from Alzheimer disease brain tissue. J. Neurochem. 61, 1916–1926.
Shapira R., Austin G.E., Mirra S.S. 1988. Neuritic plaque amyloid in Alzheimer’s disease is highly racemized. J. Neurochem. 50, 69–74.
Tomiyama T., Asano S., Furiya Y., Shirasawa T., Endo N., Mori H. 1994. Racemization of Asp23 residue affects the aggregation properties of Alzheimer amyloid beta protein analogues. J. Biol. Chem. 269, 10205–10208.
Schilling S., Zeitschel U., Hoffmann T., Heiser U., Francke M., Kehlen A., Holzer M., Hutter-Paier B., Prokesch M., Windisch M., Jagla W., Schlenzig D., Lindner C., Rudolph T., Reuter G., Cynis H., Montag D., Demuth H.U., Rossner S. 2008. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer’s disease-like pathology. Nat. Med. 14, 1106–1111.
Milton N.G. 2001. Phosphorylation of amyloid-beta at the serine 26 residue by human cdc2 kinase. Neuroreports. 12, 3839–3844.
Kumar S., Rezaei-Ghaleh N., Terwel D., Thal D.R., Richard M., Hoch M., Mc Donald J.M., Wullner U., Glebov K., Heneka M.T., Walsh D.M., Zweckstetter M., Walter J. 2011. Extracellular phosphorylation of the amyloid beta-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer’s disease. EMBO J. 30, 2255–2265.
Querfurth H.W., LaFerla F.M. 2010. Alzheimer’s disease. N. Engl. J. Med. 362, 329–344.
Fossati S., Todd K., Sotolongo K., Ghiso J., Rostagno A. 2013. Differential contribution of isoaspartate posttranslational modifications to the fibrillization and toxic properties of amyloid beta and the Asn23 Iowa mutation. Biochem. J. 456, 347–360.
Shimizu T., Watanabe A., Ogawara M., Mori H., Shirasawa T. 2000. Isoaspartate formation and neurodegeneration in Alzheimer’s disease. Arch. Biochem. Biophys. 381, 225–234.
Xin F., Radivojac P. 2012. Post-translational modifications induce significant yet not extreme changes to protein structure. Bioinformatics. 28, 2905–2913.
Kuo Y.M., Webster S., Emmerling M.R., De Lima N., Roher A.E. 1998. Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of A beta peptides of Alzheimer’s disease. Biochim. Biophys. Acta. 1406, 291–298.
Tekirian T.L., Yang A.Y., Glabe C., Geddes J.W. 1999. Toxicity of pyroglutaminated amyloid beta-peptides 3(pE)-40 and -42 is similar to that of Abeta1-40 and -42. J. Neurochem. 73, 1584–1589.
Kummer M.P., Hermes M., Delekarte A., Hammerschmidt T., Kumar S., Terwel D., Walter J., Pape H.C., Konig S., Roeber S., Jessen F., Klockgether T., Korte M., Heneka M.T. 2011. Nitration of tyrosine 10 critically enhances amyloid beta aggregation and plaque formation. Neuron. 71, 833–844.
Indeykina M.I., Popov I.A., Kozin S.A., Kononikhin A.S., Kharybin O.N., Tsvetkov P.O., Makarov A.A., Nikolaev E.N. 2011. Capabilities of MS for analytical quantitative determination of the ratio of alpha- and betaAsp7 isoforms of the amyloid-beta peptide in binary mixtures. Anal. Chem. 83, 3205–3210.
Tsvetkov P.O., Popov I.A., Nikolaev E.N., Archakov A.I., Makarov A.A., Kozin S.A. 2008. Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer’s disease amyloid beta(1-16) peptide. ChemBioChem. 9, 1564–1567.
Kozin S.A., Cheglakov I.B., Ovsepyan A.A., Telegin G.B., Tsvetkov P.O., Lisitsa A.V., Makarov A.A. 2013. Peripherally applied synthetic peptide isoAsp7-Abeta(1-42) triggers cerebral beta-amyloidosis. Neurotox. Res. 24, 370–376.
Toropygin I.Y., Kugaevskaya E.V., Mirgorodskaya O.A., Elisseeva Y.E., Kozmin Y.P., Popov I.A., Nikolaev E.N., Makarov A.A., Kozin S.A. 2008. The N-domain of angiotensin-converting enzyme specifically hydrolyzes the Arg-5-His-6 bond of Alzheimer’s Abeta-(1-16) peptide and its isoAsp-7 analogue with different efficiency as evidenced by quantitative matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 22, 231–239.
Kumar S., Wirths O., Theil S., Gerth J., Bayer T.A., Walter J. 2013. Early intraneuronal accumulation and increased aggregation of phosphorylated Abeta in a mouse model of Alzheimer’s disease. Acta Neuropathol. 125, 699–709.
Kulikova A.A., Tsvetkov P.O., Indeykina M.I., Popov I.A., Zhokhov S.S., Golovin A.V., Polshakov V.I., Kozin S.A., Nudler E., Makarov A.A. 2014. Phosphorylation of Ser8 promotes zinc-induced dimerization of the amyloid-beta metal-binding domain. Mol. Biosyst. 10, 2590–2596.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Kulikova, A.A. Makarov, S.A. Kozin, 2015, published in Molekulyarnaya Biologiya, 2015, Vol. 49, No. 2, pp. 249–263.
Rights and permissions
About this article
Cite this article
Kulikova, A.A., Makarov, A.A. & Kozin, S.A. Roles of zinc ions and structural polymorphism of β-amyloid in the development of Alzheimer’s disease. Mol Biol 49, 217–230 (2015). https://doi.org/10.1134/S0026893315020065
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893315020065