Abstract
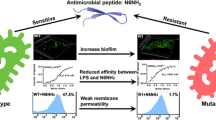
Augmented resistance against antimicrobial peptides in pathogenic bacteria has become a serious concern leading to increased bacterial virulence, which limits the applications of these peptides as biopreservatives. Nisin and Human Neutrophil Peptide-1 (HNP-1) are the peptides with biopreservative/clinical and physiological relevance, respectively. In the present study, certain aspects of resistance development against these peptides were investigated in Enterococcus faecalis. Three strains of E. faecalis with different levels of resistance against nisin were selected. Also, wild-type bacteria were challenged with low and high doses of HNP-1. Using in silico analysis, we identified a two-component cationic antimicrobial peptide (CAMP) sensing system in E. faecalis. Gene expression analysis revealed this system to be activated in nisin-resistant variants with increased net positive charge on bacterial cell surface. Cytochrome c assay and thin layer chromatography of the lipids derived from these bacterial strains corroborate increased cell surface positive charge upon resistance acquisition. The identified sensing system was not found to be activated in HNP-1-challenged cells, although an increased positive charge was found on the surface of these cells, indicating the possibility of more than one CAMP sensing system in E. faecalis. Both cell surface hydrophobicity and biofilm formation were increased in nisin-resistant strains, although biofilm formation was found to remain unaffected in HNP-1 challenged cells, which might be related to their unaltered expression levels of dltA gene.





Similar content being viewed by others
REFERENCES
Bader, M.W., Sanowar, S., Daley, M.E., Schneider, A.R., Cho, U., Xu, W., Klevit, R.E., Moual, H.L., and Miller, S.I., Recognition of antimicrobial peptides by a bacterial sensor kinase, Cell, 2005, vol. 122, pp. 461–472.
Baldassarri, L., Cecchini, R., Bertuccini, L., Ammen-dolia, M.G., Iosi, F., Arciola, C.R., Montanaro, L., Di-Rosa, R., Gherardi, G., Dicuonzo, G., and Orefici, G., Enterococcus spp. produces slime and survives in rat peritoneal macrophages, Med. Microbiol. Immun., 2001, vol. 190, pp. 113–120.
Bligh, E.G. and Dyer, W.J., A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol., 1959, vol. 37, pp. 911–917.
Brodsky, I.E. and Gunn, J.S., A bacterial sensory system that activates resistance to innate immune defenses: potential targets for antimicrobial therapeutics, Mol. Interv., 2005, vol. 5, pp. 335–337.
Cabo, M.L., Murado, M.A., González, M.P., and Pastoriza, L., A method for bacteriocin quantification, J. Appl. M-icrobiol., 1999, vol. 87, pp. 907–914.
Dabirian, S., Taslimi, Y., Zahedifard, F., Gholami, E., Doustdari, F., Motamedirad, M., Khatami, S., Azadmanesh, K., Nylen, S., and Rafati, S., Human neutrophil peptide-1 (HNP-1): a new anti-leishmanial drug candidate, PLoS Negl. Trop. Dis., 2013, vol. 7, p. e2491.
De la Fuente-Núñez, C., Reffuveille, F., Fernández, L., and Hancock, R.E., Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies, Curr. Opin. Microbiol., 2013, vol. 16, pp. 580–589.
Donlan, R.M., Biofilms and device-associated infections, Emerg. Infect. Dis., 2001, vol. 7, pp. 277–281.
Drenkard, E., Antimicrobial resistance of Pseudomonas aeruginosa biofilms, Microbes Infect., 2003, vol. 5, pp. 1213–1219.
Fabretti, F., Theilacker, C., Baldassarri, L., Kaczynski, Z., Kropec, A., Holst, O., and Huebner, J., Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides, Infect. Immun., 2006, vol. 74, pp. 4164–4171.
Gera, J.F. and Lichtenstein, A., Human neutrophil peptide defensins induce single strand DNA breaks in target cells, Cell Immunol., 1991, vol. 138, pp. 108–120.
Giaouris, E., Chapot-Chartier, M.P., and Briandet, R., Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties, Int. J. Food Microbiol., 2009, vol. 131, pp. 2–9.
Gillis, R.J., White, K.G., Choi, K.H., Wagner, V.E., S-chweizer, H.P., and Iglewski, B.H., Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms, Antimicrob. Agents Chemother., 2005, vol. 49, pp. 3858–3867.
Gilmore, M.S., Clewell, D.B., Ike, Y., and Shankar, N., Enterococci: from commensals to leading causes of drug resistant infection [internet]. Boston: Massachusetts Eye and Ear Infirmary. 2014. https://www.ncbi.nlm.nih.gov/ books/NBK190424/.
Høiby, N., Bjarnsholt, T., Givskov, M., Molin, S., and Ciofu, O., Antibiotic resistance of bacterial biofilms, Int. J. Antimicrob Agents, 2010, vol. 35, pp. 322–332.
Jacquet, T., Cailliez-Grimal, C., Borges, F., Gaiani, C., Francius, G., Duval, J.F., Waldvogel, Y., and Revol-Junelles, A.M., Surface properties of bacteria sensitive and resistant to the class IIa carnobacteriocin Cbn BM1, J. A-ppl. Microbiol., 2012, vol. 112, pp. 372–382.
Jolivet-Gougeon, A. and Bonnaure-Mallet, M., Biofilms as a mechanism of bacterial resistance, Drug Discov. Today Technol., 2014, vol. 11, pp. 49–56.
Kang, J., Wiedmann, M., Boor, K.J., and Bergholz, T.M., VirR-mediated resistance of Listeria monocytogenes against food antimicrobials and cross-protection induced by exposure to organic acid salts, Appl. Environ. Microbiol., 2015, vol. 81, pp. 4553–4562.
Kovács, M., Halfmann, A., Fedtke, I., Heintz, M., Peschel, A., Vollmer, W., Hakenbeck, R., and Bruckner, R., A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumonia, J. Bacteriol., 2006, vol. 188, pp. 5797–5805.
Krasowska, A. and Sigler, K., How microorganisms use hydrophobicity and what does this mean for human needs?, Front. Cell. Infect. Microbiol., 2014, vol. 4, p. 112.
Kristian, S.A., Datta, V., Weidenmaier, C., Kansal, R., Fedtke, I., Peschel, A., Gallo, R.L., and Nizet, V., D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion, J. Bacteriol., 2005, vol. 187, pp. 6719–6725.
Kumariya, R., Sood, S.K., Rajput, Y.S., and Garsa, A.K., Gradual pediocin PA-1 resistance in Enterococcus faecalis confers cross-protection to diverse pore-forming cationic antimicrobial peptides displaying changes in cell wall and mannose PTS expression, Ann. Microbiol., 2014, vol. 65, pp. 721–732.
Kumariya, R., Sood, S.K., Rajput, Y.S., Saini, N., and Garsa, A.K., Increased membrane surface positive charge and altered membrane fluidity leads to cationic antimicrobial peptide resistance in Enterococcus faecalis, Biochim. Biophys. Acta Biomembr., 2015, vol. 1848, pp. 1367–1375.
Lather, P., Mohanty, A.K., Jha, P., and Garsa, A.K., Contribution of cell surface hydrophobicity in the resistance of Staphylococcus aureus against antimicrobial agents, Biochem. Res. Int., 2016.
Li, M., Cha, D.J., Lai, Y., Villaruz, A.E., Sturdevant, D.E., and Otto, M., The antimicrobial peptide-sensing system aps of Staphylococcus aureus, Mol. Microbiol., 2007, vol. 66, pp. 1136–1147.
Li, M., Lai, Y., Villaruz, A.E., Cha, D.J., Sturdevant, D.E., and Otto, M., Gram-positive three-component antimicrobial peptide-sensing system, Proc. Natl. Acad. Sci. U. S. A., 2007, vol. 104, pp. 9469–9474.
Mah, T.F., Biofilm-specific antibiotic resistance, Future Microbiol., 2012, vol. 7, pp. 1061–1072.
Mandin, P., Fsihi, H., Dussurget, O., Vergassola, M., Milohanic, E., Toledo-Arana, A., Lasa, I., Johanssan, J., and Cossart, P., VirR, a response regulator critical for Listeria monocytogenes virulence, Mol. Microbiol., 2005, vol. 57, pp. 1367–1380.
Matsuo, M., Oogai, Y., Kato, F., Sugai, M., and Komatsuzawa, H., Growth-phase dependence of susceptibility to antimicrobial peptides in Staphylococcus aureus, Microbiology (SGM), 2011, vol. 157, pp. 1786–1797.
Meehl, M., Herbert, S., Götz, F., and Cheung, A., Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus, Antimicrob. Agents Chemother., 2007, vol. 51, pp. 2679–2689.
Mehla, J. and Sood, S.K., In vitro substantiation of dose dependent resistance and cross resistance phenomenon in E. faecalis against pore forming antimicrobial peptides using a PDA based in vitro colorimetric assay, Appl. Environ. Microbiol., 2011, vol. 77, pp. 786–793.
Mehta, S., Cuirolo, A.X., Plata, K.B., Riosa, S., Silver-man, J.A., Rubio, A., Rosato, R.R., and Rosato, A.E., VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus, Antimicrob. Agents Chemother., 2012, vol. 56, pp. 92–102.
Mirani, Z.A., Fatima, A., Urooj, S., Aziz, M., Khan, M.N., and Abbas, T., Relationship of cell surface hydrophobicity with biofilm formation and growth rate: a study on Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli, Iran. J. Basic Med. Sci., 2018, vol. 21, p. 760.
Molin, S. and Tolker-Nielsen, T., Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure, Curr. Opin. Biotechnol., 2003, vol. 14, pp. 255–261.
Mulcahy, H., Charron-Mazenod, L., and Lewenza, S., Extracellular DNA chelates cations and induces antimicrobial resistance in Escherichia coli biofilms, PLoS Pathog., 2008, vol. 4, p. e1000213.
Neuhaus, F.C. and Baddiley, J., A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria, Microbiol Mol. Biol. Rev., 2003, vol. 67, pp. 686–723.
Pearson, W.R., An introduction to sequence similarity (“homology”) searching, Curr. Protoc. Bioinformatics, 2013, vol. 42, pp. 3–1.
Peschel, A., Jack, R.W., Otto, M., Collins, L.V., Staubitz, P., Nicholson, G., Kalbacher, H., Nieuwenhuizen, W.F., Jung, G., Tarkowski, A., Kessel, K., and Strijp, J., Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine, J. Exp. Med., 2001, vol. 193, pp. 1067–1076.
Peschel, A., Otto, M., Jack, R.W., Kalbacher, H., Jung, G., and Gotz, F., Inactivation of the dlt Operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides, J. Biol. Chem., 1999, vol. 274, pp. 8405–8410.
Reifsteck, F., Wee, S., and Wilkinson, B.J., Hydrophobicity–hydrophilicity of staphylococci, J. Med. Microbiol., 1987, vol. 24, pp. 65–73.
Shin, J.M., Gwak, J.W., Kamarajan, P., Fenno, J.C., Rickard, A.H., and Kapila, Y.L., Biomedical applications of nisin, J. Appl. Microbiol., 2016, vol. 120, pp. 1449–1465.
Soto, S.M., Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm, Virulence, 2013, vol. 4, pp. 223–229.
Stark, M., Liu, L.P., and Deber, C.M., Cationic hydrophobic peptides with antimicrobial activity, Antimicrob. Agents Chemother., 2002, vol. 46, pp. 3585–3590.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contribution of the entire faculty and technical staff of Animal Biochemistry Division, ICAR-NDRI, Karnal, India.
Funding
This work was supported by Inspire programme, Department of Science and Technology (DST), India and ICAR-National Dairy Research Institute, Karnal, India. Author Dr. Ram Krishan Saini is thankful to Indian Council of Medical Research (ICMR), India for their support in terms of Ph.D fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
AUTHORS’ CONTRIBUTION
Neha Saini contributed to the methodology, statistical analysis and manuscript writing. Ram Krishan Saini and Surya Kant Verma contributed to the data acquisition. Shiv Kumar Sood contributed in conceptualization and supervision.
Rights and permissions
About this article
Cite this article
Saini, N., Saini, R.K., Verma, S.K. et al. Some Aspects of Resistance Development against Nisin and Human Neutrophil Peptide-1 in Enterococcus faecalis. Microbiology 92, 704–714 (2023). https://doi.org/10.1134/S0026261722601646
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722601646