Abstract
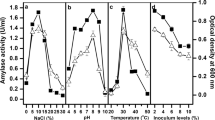
Halotolerant bacterium Bacillus vallismortis (HQ992818) was isolated from saltern sediments in India, and produced significantly high levels extracellular amylase. A detailed investigation on the culture conditions including period of incubation, media pH, and inoculum size in addition to different sources of carbon and nitrogen, metal ions, NaCl, and amino acids was carried out for optimized production. Maximum amylase production (62 U/mL) was attained after 26 h of incubation. The optimized conditions for maximal production of amylase were found to be 1% NaCl, pH 8, temp 37°C, 1% starch, 1% sodium nitrate, phenyl alanine (0.01%) and calcium chloride (10 mM). The biochemical characteristics of the extracellular amylase were studied with respect to change in temperature, pH and metal ions. The enzyme was found to be optimally active in the temperature range of 40–70°C and pH 8. Activation of the enzyme by Ca2+ (135%), Fe2+ (113%) and Mg2+ (109%) occurred at 5 mM concentration and strongly inhibited by Hg2+, Zn2+ and Mn2+ occurred at 10 mM. Significant compatibility of the enzyme with the commercial laundry detergents and the results of washing performance test confirmed its effectiveness. Available data on the optimized culture conditions enables for easily adaptable setup of large scale production of the enzyme for use in detergent formulations.
Similar content being viewed by others
References
Rajagopalan, G. and Krishnan, C., Alpha amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate, Biores. Technol., 2008, vol. 99, pp. 3044–3050.
Kondepudi, K.K. and Chandra, T.S., Production of surfactant and detergent-stable, halophilic and alkalitolerant α-amylase by a moderately halophilic Bacillus sp. strain TSCVKK, Appl. Microbiol. Biotechnol., 2008, vol. 77, pp 1023–1031.
Mageswari, A., Parthiban, S., Suganthi, C., Karthikeyan, S., Babu, S., and Gothandam, K.M., Optimization and immobilization of amylase obtained from halotolerant bacteria isolated from solar salterns, J. Genet. Eng. Biotechnol., 2012, vol. 10, pp. 201–208.
Hough, D.W. and Danson, M.J., Extremozymes, Curr. Opin. Chem. Biol., 1999, vol. 1, pp. 39–46.
Patel, R., Dodia, M., and Singh, S.P., Extracellular alkaline protease from a haloalkaliphilic Bacillus sp: production and optimization, Proc. Biochem., 2005, vol. 40, pp. 3569–3575.
Ollivier, B., Caumette, P., Garcia, J.L., and Mah, R.A., Anaerobic bacteria from hypersaline environments, Microbiol. Rev., 1994, vol. 58, pp. 27–38.
Ventosa, A. and Nieto, J.J., Biotechnological applications and potentialities of halophilic microorganisms, World J. Microbiol. Biotechnol., 1995, vol. 11, pp. 85–94.
Amoozegara, M.A., Malekzadeha, F., Khursheed, A., and Malik, Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2, J. Microbiol. Methods, 2003, vol. 52, pp. 353–359.
Prakash, B., Vidyasagar, M., Madhukumar, M.S., Muralikrishna, G., and Sreeramulu, K., Production, purification, and characterization of two extremely halotolerant, thermostable and alkali-stable alpha amylases from Chromohalobacter sp. TVSP 101, Proc. Biochem., 2009, vol. 44, pp. 210–215.
Jaya Prakash Goud, M., Suryam, A., Lakshmipathi, V., and Singara Charya, M.A., Extracellular hydrolytic enzyme profiles of certain South Indian basidiomycetes, Afr. J. Biotechnol., 2009, vol. 8, pp. 354–360.
Babu, K.R. and Satyanarayana, T., Parametric optimization of extracellular α-amylase production by thermophilic Bacillus coagulans, Folia Microbiol., 1993, vol. 38, pp. 77–80.
Miller, G.L., Use of dinitrosalicyclic acid reagent for determination of reducing sugar, J. Anal. Chem., 1959, vol. 31, pp. 426–428.
Sambrook, J., Fritsch, E.F., and Maniatis, T., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor: Cold Spring Harbor Laboratory Press, vol. 3.
Michael, S., Roberts., Nakumura, L.K., and Choa, F.M., Bacillus vallismortis sp. nov., close relative of Bacillus subtilis, isolated from soil in Death Valley, California, Int. J. Syst. Bacteriol., 1996, vol. 46, pp. 470–475.
Khire, J.M. and Pant, A., Thermostable salt-tolerant amylase from Bacillus sp., World J. Microbiol. Biotechnol., 1992, vol. 8, pp. 167–170.
Vijayabaskar, P., Jayalakshmi, D., and Shankar, T., Amylase production by moderately halophilic Bacillus cereus in solid state fermentation, Afr. J. Microbiol. Res., 2012, vol. 6, pp. 4918–4926.
Jetendra, K., Roy., Sudhir, K., Rai., Ashis, K., and Mukherjee, Characterization and application of a detergent-stable alkaline α-amylase from Bacillus subtilis strain AS-S01a, J. Biol. Macromolec., 2012, vol. 50, pp. 219–229.
Burhan, A., Nisa, U., Gokhan, C., Omer, C., Ashabil, A., and Osman, G., Enzymatic properties of a novel thermophilic, alkaline and chelator resistant amylase from an alkalophilic Bacillus sp. isolate ANT-6, Proc. Biochem., 2003, vol. 38, pp. 1397–1403.
Jin, B., Van-Leeuwen, J.H., and Patel, B., Mycelial morphology and fungal protein production from starch processing wastewater in submerged cultures of Aspergillus oryzae, Proc. Biochem., 1999, vol. 34, pp. 335–340.
Asgher, M., Javaid Asad, M., Rahman, S.U., and Legge, R.L., A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing, J. Food Eng., 2007, vol. 79, pp. 950–955.
Sivakumar, T., Shankar, T., Vijayabaskar, P., Muthukumar, J., and Nagendrakannan, E., Amylase production using Bacillus cereus isolated from a vermicompost site, Intl. J. Microbiol. Res., 2012, vol. 3, pp. 117–123.
Haseltine, C., Rolfsmeier, M., and Blum, P., The glucose effect and regulation of α-amylase synthesis in the hyperthermophilic archeon Sulfolobus solfataricus, J. Bacteriol., 1996, vol. 178, pp. 945–950.
Mukesh kumar, D.J., Andal Priyadharshini, D., Suresh, K., Saranya, G.M., Rajendran, K., and Kalaichelvan, Production, purification and characterization of α-amylase and alkaline protease by Bacillus sp. HPE10 in a concomitant production medium, Asian J. Plant Sci. Res., 2012, vol. 2, pp. 376–382.
Thippeswamy, S., Girigowda, K., and Mulimani, V.H., Isolation and identification of α-amylase producing Bacillus sp. from dhal industry waste, Indian J. Biochem. Biophys., 2006, vol. 43, pp. 295–298.
Rasooli, I., Astaneh, S.D.A., Borna, H., and Barchini, K.A., Thermostable α-amylase producing natural variant of Bacillus spp. isolated from soil in Iran, Am. J. Agri., Bio. Sci., 2008, vol. 3, pp. 591–596.
Hamilton, L.M., Kelly, C.T., and Fogarty, W.M., Purification and properties of the raw starch degrading α-amylase of Bacillus sp. IMD 434, Biotech. Lett., 1999, vol. 21, pp. 111–115.
Goyal, N., Gupta, J.K., and Soni, S.K., A novel raw starch digesting thermostable α-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch, Enzyme Microb., vol. 37, pp. 723–734.
Gupta, R., Gigras, P., Mohapatra, H., Goswami, V.K., and Chauhan, B., Microbial α-amylase: a biotechnological perspective, Proc. Biochem., 2003, vol. 38, pp. 1599–1616.
Shafiei, M., Ziaee A.A., and Amoozegar M.A., Purification and biochemical characterization of a novel SDS and surfactant stable, raw starch digesting, and halophilic α-amylase from a moderately halophilic bacterium, Nesterenkonia sp. strain F, Proc. Biochem., 2010, vol. 45, pp. 694–699.
Cordeiro, C.A.M., Martins, M.L.L., and Luciano, A.B., Production and properties of α-amylase from thermophilic Bacillus sp., Braz. J. Microbiol., 2002, vol. 33, pp. 57–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Suganthi, C., Mageswari, A., Karthikeyan, S. et al. Insight on biochemical characteristics of thermotolerant amylase isolated from extremophile bacteria Bacillus vallismortis TD6 (HQ992818). Microbiology 84, 210–218 (2015). https://doi.org/10.1134/S0026261715020162
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261715020162