Abstract
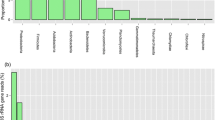
The structural changes in the bacterial community composition of the Galitskii Mokh mesotrophic peatland (Tver oblast) after the 2010 wildfire were analyzed. Burned sites demonstrated an increase in peat water pH and mineralization, with elevated nitrate and sulfate concentrations, which resulted in doubling of the numbers of culturable sulfate reducers. Although the rate of methane oxidation by peat samples from burned sites decreased, methane emission did not increase. Enumeration of bacterial phylogenetic groups by fluorescent in situ hybridization revealed doubling of the numbers of Alphaproteobacteria and Bacteroidetes in burned areas compared to an undamaged site, while a reverse pattern was observed for Deltaproteobacteria and Planctomycetes. Comparative analysis of the 16S rRNA clone libraries revealed shifts in the bacterial community composition in the peat from burned sites. Slowly growing bacteria of the phyla Verrucomicrobia and Planctomycetes were replaced by fast-growing colonizers from the Proteobacteria. The originally diverse Actinobacterial community was replaced by the one dominated by the phylotypes related to thermophilic Aciditerrimonas ferrireducens. In the Acidobacteria, a typical group of peat-inhabiting bacteria, the subgroups 3, 6, and 17 were replaced by subgroups 7 and 10. These changes in the structure of peatland microbial communities could result in disturbances of some biospheric functions of these ecosystems.
Similar content being viewed by others
References
Zavarzin, G.A. and Dedysh, S.N., Microbial processes in bog ecosystems: effect on environment and climate, in Izmenenie okruzhayushchei sredy i klimata: prirodnye i svyazannye s nimi tekhnogennye katastrofy. T. 4: Protsessy v biosfere: izmeneniya pochvenno-rastitel’nogo pokrova i territorial’nykh vod RF, krugovorot veshchestv pod vliyaniem global’nykh izmenenii klimata i katastroficheskikh protsessov (Environmental and Climatic Changes: Natural and Associated Technogenic Catastrophes. Vol. 4. Biospheric Processes: Changes in RF Soil, Vegetable Cover, and Territorial Waters Caused by Global Climatic Changes and Catastrophic Processes), Zavarzin, G.A. and Kudeyarov, V.N., Eds., Pushchino: IPC, 2008, pp. 80–96.
Fritze, H. and Pietikainen, J., Recovery of soil microbial biomass and activity from prescribed burning, Can. J. Forest Res., 1993, vol. 23, pp. 1286–1290.
Pietikainen, J. and Fritze, H., Microbial biomass and activity in the humus layer following burning: short-term effects of two different fires, Can. J. Forest Res., 1993, vol. 23, pp. 1275–1285.
Acea, M.J. and Carballas, T., Changes in physiological groups of microorganisms in soil following wildfire, FEMS Microbiol. Ecol., 1996, vol. 20, pp. 33–39.
Hernandez, T., Garcia, C., and Reinhardt, I., Short-term effect of wildfire on the chemical, biochemical, and microbiological properties of Mediterranean pine forest soils, Biol. Fertil. Soils, 1997, vol. 25, pp. 109–116.
Pietikainen, J., Hiukka, R., and Fritze, H., Does short-term heating of forest humus change its properties as a substrate for microbes?, Soil Biol. Biochem., 2000, vol. 32, pp. 277–288.
Prietro-Fernandez, A., Acea, M.J., and Carballas, T., Soil microbial and extractable — and N after wildfire, Biol. Fertil. Soils, 1998, vol. 27, pp. 132–142.
Saa, A., Trasar-Cepeda, M.C., and Carballas, T., Soil phosphorus status and phosphomonoesterase activity of recently burnt and unburnt soil following laboratory incubation, Soil Biol. Biochem., 1998, vol. 30, pp. 419–428.
Vázquez, F.J., Acea, M.J., and Carballas, T., Changes in physiological groups of microorganisms in soil following wildfire, Microb. Ecol., 1993, vol. 13, pp. 93–104.
Bogorodskaya, A.V., Ivanova, G.A., and Tarasov, P.A., Post-fire transformation of the microbial complexes in soils of larch forests in the Lower Angara River region, Euras. Soil Sci., 2011, vol. 1, pp. 49–55.
Verma, S. and Jayakumar, S., Impact of forest fire on physical, chemical and biological properties of soil: a review, Proc. Int. Acad. Ecol. Environ. Sci., 2012, vol. 2, no. 3, pp. 168–176.
Akhmet’eva, N.P., Belova, S.E., Dzhamalov, R.G., Kulichevskaya, I.S, Lapina, E.E., and Mikhailova, A.V., Natural post-fire bog recovery, Water Res., 2014, vol. 41, no. 4, pp. 353–363.
Zoltai, S.C., Morrissey, L.A., Livingston, G.P., and de Groot, W.J., Effects of fires on carbon cycling in North American boreal peatlands, Environ. Rev., 1998, vol. 6, no. 1, pp. 13–24.
Wieder, R.K., Scott, K.D., Kamminga, K., Vile, M.A., Vittd, H., Bone, T., Xu, B., Benscoter, B. W., and Bhatti, J.S., Postfire carbon balance in boreal bogs of Alberta, Canada, Global Change Biol., 2009, vol. 15, pp. 63–81.
Grosse, G., Harden, J., Turetsky, M., McGuire, A.D., Camill, P., Tarnocia, C., et al., Vulnerability of high-latitude soil organic carbon in North America to disturbance, J. Geophys. Res., 2011, vol. 116, G00K06. doi: 10.1029/2010JG001507
Zavarzin, G.A., Ombrophiles—inhabitants of the plains, Priroda (Moscow, Russ. Fed.), 2009, no. 6, pp. 3–14.
Glagolev, M., Kleptsova, I., Filippov, I., Maksyutov, S., and Machida, T., Regional methane emission from West Siberia mire landscapes, Environ. Res. Lett., 2011, vol. 6, no. 4, 045214. DOI: 10.1088/1748-9326/6/4/045214
Kuznetsov, S.I. and Dubinina, G.A., Metody izucheniya vodnykh mikroorganizmov (Methods for Analysis of Natural Waters), Moscow: Nauka, 1989.
Pankratov, T.A., Belova, S.E., and Dedysh, S.N., Evaluation of the phylogenetic diversity of prokaryotic microorganisms in Sphagnum peat bogs by means of fluorescence in situ hibridization (FISH), Microbiology (Moscow), 2005, vol. 74, no. 6, pp. 722–728.
Dedysh, S.N., Pankratov, T.A., Belova, S.E., Kulichevskaya, I.S., and Liesack, W., Phylogenetic analysis and in situ identification of Bacteria community composition in an acidic sphagnum peat bog, Appl. Environ. Microbiol., 2006, vol. 72, no. 3, pp. 2110–2117.
Weisburg, W.G., Barns, S.M., Pelletier, D.A., and Lane, D.J., 16S ribosomal DNA amplification for phylogenetic study, J. Bacteriol., 1991, vol. 173, pp. 697–703.
Danilova, O.V. and Dedysh, S.N., Abundance and diversity of methanotrophic Gammaproteobacteria in northern wetlands, Microbiology (Moscow), 2014, vol. 83, nos. 1–2, pp. 67–76.
Stams, A.J.M., Plugge, C.M., de Bok, F.A.M., van Houten, B.H.G.W., Lens, P., and Dijkman, H., Metabolic interactions in methanogenic and sulfate-reducing bioreactors, Water Sci. Technol., 2005, vol. 52, pp. 13–20.
Kulichevskaya, I.S., Pankratov, T.A., and Dedysh, S.N., Detection of representatives of the Planctomycetes in Sphagnum peat bogs by molecular and cultivation approaches, Microbiology (Moscow), 2006, vol. 75, no. 3, pp. 329–335.
Serkebaeva, Y.M., Kim, Y., Liesack, W., and Dedysh, S.N., Pyrosequencing-based assessment of the Bacteria diversity in surface and subsurface peat layers of a northern wetland, with focus on poorly studied phyla and candidate divisions, Plos One, 2013, vol. 8, no. 5: e63994. doi: 10.1371/journal.pone.0063994
Itoh, T., Yamanoi, K., Kudo, T., Ohkuma, M., and Takashina T., Aciditerrimonas ferrireducens gen. nov., sp. nov., an iron-reducing thermoacidophilic actinobacterium isolated from a solfataric field, Int. J. Syst. Evol. Microbiol., 2011, vol. 61, pp. 1281–1285.
Spieck, E., Hartwig, C., McCormack, I., Maixner, F., Wagner, M., Lipski, A., and Daims, H., Selective enrichment and molecular characterization of a previously uncultured Nitrospira-like bacterium from activated sludge, Environ. Microbiol., 2006, vol. 8, no. 3, pp. 405–415.
Izumi, H., Nunoura, T., Miyazaki, M., Mino, S., Toki, T., Takai, K., Sako, Y., Sawabe, T., and Nakagawa, S., Thermotomaculum hydrothermale gen. nov., sp. nov., a novel heterotrophic thermophile within the phylum Acidobacteria from a deep-sea hydrothermal vent chimney in the Southern Okinawa Trough, Extremophiles, 2012, vol. 16, pp. 245–253.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belova, S.E., Kulichevskaya, I.S., Akhmet’eva, N.P. et al. Shifts in a bacterial community composition of a mesotrophic peatland after wildfire. Microbiology 83, 813–819 (2014). https://doi.org/10.1134/S0026261714060022
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261714060022