Abstract
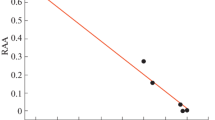
The rate constant of the reaction of methano- and cyclopentenofullerenes (С60R) with a peroxyl radical (PhCH(OO•)CH3) was measured in a model system of the radical-chain oxidation of ethylbenzene. Compounds with a succinimide dodecenoate substituent bonded with the fullerene molecule via the ‒С(О)(СН2)n– or –(СН2)n– groups (n = 1–5) were studied. The reactivity of С60R with respect to the ethylbenzene peroxyl radical was shown to increase relative to that of unsubstituted fullerene С60. Methanofullerenes were more reactive with the peroxyl radical than cyclopentenofullerenes. The rate constant of the reaction of С60R with PhCH(OO•)CH3 decreased when the number of methylene groups (n) increased. Quantum chemical modeling showed that the substituent atoms form hydrogen bonds with the peroxyl radical in the transition states of addition at the atoms of the fullerenyl moiety of methanofullerene that are nearest to the substituent. As a result, the energy barrier of the peroxyl radical addition to fullerene decreases. In the cases when succinimide dodecenoate lies far from the fullerene molecule, the reactivity of methano- and cyclopentenofullerenes with respect to the peroxyl radical decreases.





Similar content being viewed by others
REFERENCES
Grebowski, J., Konopko, A., Krokosz, A., DiLabio, G.A., and Litwinienko, G., Free Radicals Biol. Med., 2020, vol. 160, p. 734.
Volkov, V.A., Voronkov, M.V., Sazhina, N.N., Kurilov, D.V., Vokhmyanina, D.V., Yamskova, O.V., Martirosyan, Yu.Ts., Atroshenko, D.L., Martirosyan, L.Yu., and Romanova, V.S., Kinet. Catal., 2021, vol. 62, no. 3, p. 395.
Sharoyko, V.V., Ageev, S.V., Podolsky, N.E., Petrov, A.V., Litasova, E.V., Vlasov, T.D., Vasina, L.V., Murin, I.V., Piotrovskiy, L.B., and Semenov, K.N., J. Mol. Liq., 2021, vol. 323, p. 114990.
Enes, R.F., Tomé, A.C., Cavaleiro, J.A.S., Amorati, R., Fumo, M.G., Pedulli, G.F., and Valgimigli, L., Chem. Eur. J., 2006, vol. 12, no. 17, p. 4646.
Cataldo, F., Chem. Phys. Lipids, 2010, vol. 163, no. 6, p. 524.
Matsubayashi, K., Goto, T., Kyoko, TogayaK., Kokubo, K., and Oshima, T., Nanoscale Res. Lett., 2008, vol. 3, no. 8, p. 237.
Yakupova, L.R., Sakhautdinov, I.M., Malikova, R.N., and Safiullin, R.L., Kinet. Catal., 2019, vol. 60, no. 1, p. 21.
Yakupova, L.R., Fattakhov, A.Kh., Gimadieva, A.R., Safiullin, R.L., and Sakhautdinova, R.A., Kinet. Catal., 2013, vol. 54, no. 3, p. 279.
Yakupova, L.R., Ivanova, A.V., Safiullin, R.L., Gimadieva, A.R., Chernyshenko, Yu.N., Mustafin, A.G., and Abdrakhmanov, I.B., Russ. Chem. Bull., 2010, vol. 59, no. 3, p. 517.
Yakupova, L.R., Proskuryakov, S.G., Zaripov, R.N., Rameev, Sh.R., and Safiullin, R.L., Butlerov. Soobshch., 2011, vol. 28, no. 19, p. 71.
Kulitski, Z.I., Terman, L.M., Tsepalov, V.F., and Shlyapintokh, V.Ya., Izv. Akad. Nauk SSSR, Ser. Khim., 1963, no. 2, p. 253.
Cand. Sci. (Chem.) Dissertation, Ufa: UIC UFRS RAS, 2022.
Laikov, D.N. and Ustynyuk, Yu.A., Russ. Chem. Bull., 2005, vol. 54, p.820.
Perdew, J.P., Burke, K., and Ernzerhof, M., Phys. Rev. Lett., 1996, vol. 77, p. 3865.
Diniakhmetova, D.R., Friesen, A.K., and Kolesov, S.V., Int. J. Quantum Chem., 2020, vol. 120, no. 18, e26335.
Diniakhmetova, D.R., Friesen, A.K., and Kolesov, S.V., Int. J. Quantum Chem., 2016, vol. 116, no. 7, p. 489.
Sabirov, D.Sh. and Bulgakov, R.G., Comput. Theor. Chem., 2011, vol. 963, no. 1, p. 185.
Sabirov, D.Sh. and Bulgakov, R.G., Chem. Phys. Lett., 2011, vol. 506, nos. 1–3, p. 52.
Galimov, D.I., Gazeeva, D.R., Mukhamed’yarova, R.K., and Bulgakov, R.G., Vestnik BashGU, 2012, vol. 17, no. 4, p. 1671.
Denisov, E.T. and Azatyan, V.V., Ingibirovanie tsepnykh reaktsii (Inhibition of Chain Reactions), Chernogolovka: RAS, 1997.
Tsepalov V.F. and Shlyapintokh V.Ya., Kinet. Katal., 1962, vol. 3, no. 6, p. 870.
Safarova, I.V., Sharipova, G.M., Nugumanova, E.F., and Gerchikov, A.Ya., Vestnik BashGU, 2016, vol. 21, no. 1, p. 37.
Ali, S.S., Hardt, J.I., Quick, K.L., Kim-Han, J.S., Erlanger, B.F., Huang, T.T., Epstein, C.J., and Dugan, L.L., Free Rad. Biol. Med., 2004, vol. 37, no. 8, p. 1191.
Sabirov, D.Sh., Garipova, R.R., and Bulgakov, R.G., Fullerenes, Nanotubes, Carbon Nanostruct., 2015, vol. 23, no. 12, p. 1051.
Knight, B., Martín, N., Ohno, T., Ortí, E., Rovira, C., Veciana, J., Vidal-Gancedo, J., Viruela, P., Viruela, R., and Wudl, F., J. Am. Chem. Soc., 1997, vol. 119, no. 41, p. 9871.
Tumanskii, B.L., Nefedova, M.N., Bashilov, V.V., Solodovnikov, S.P., Bubnov, N.N., and Sokolov, V.I., Russ. Chem. Bull., 1996, vol. 45, p. 2865.
Godly, E.W. and Taylor, R., Pure Appl. Chem., 1997, vol. 69, p. 1411.
Funding
This study was performed in accordance with the research plan at Ufa Institute of Chemistry, Ufa Federal Research Center, Russian Academy of Sciences (topic “Mechanism and kinetic laws of oxidative transformations involving highly active intermediates in chemical and biochemical processes,” FMRS-2022-0021, 122031400255-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by L. Smolina
Abbreviations and notation: F, degree of the inhibiting action of the inhibitor; ki, initiation rate constant; k1 and k2, rate constants of chain propagation; k6, rate constant of termination of the oxidation chain by recombination of peroxyl radicals; k7, inhibition rate constant; f, stoichiometric inhibition coefficient; PhCH2CH3, oxidized substrate (ethylbenzene); PhC•(H)CH3, PhCH(OO•)CH3, alkyl and peroxyl radicals formed from the substrate; AIBN, 2,2'-azo-bis-isobutyronitrile; EB, ethylbenzene.
Rights and permissions
About this article
Cite this article
Yakupova, L.R., Diniakhmetova, D.R., Sakhautdinov, I.M. et al. Antioxidant Activity of Methano- and Cyclopentenofullerenes. Kinet Catal 63, 463–469 (2022). https://doi.org/10.1134/S0023158422050160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158422050160