Abstract
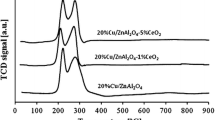
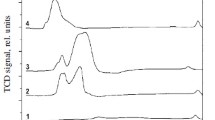
Monometallic copper and bimetallic palladium–copper catalysts supported on ZnO–Al2O3, CeO2–Al2O3 and ZrO2–Al2O3 were prepared by conventional impregnation method and tested in the methanol synthesis reaction in a gradient less reactor under elevated pressure (3.5 MPa) at 220°C. The physicochemical properties of prepared catalytic systems were studied using BET, TPR-H2, TPD-NH3, XRD, SEM-EDS and FT-IR techniques. The results of XRD and SEM-EDS measurements showed the formation of Pd–Cu alloy during the activation of bimetallic catalysts. It was found that the formed alloy was responsible for the improved activity and selectivity of catalysts in the studied reaction. Among investigated catalysts, the highest formation rate of methanol was observed with 2%Pd–20%Cu/ZnO–Al2O3 system. Based on the results of FT-IR measurements it can be concluded that hydrogen molecules adsorb dissociatively on the metallic copper surface to form hydrogen atoms, increasing the hydrogen spillover effect on the metal-support interface. In contrast, CO2 adsorb on the oxygen vacancies of the support to form carbonates, which can further undergo hydrogenation to methanol.



Similar content being viewed by others
REFERENCES
Davis, S.J., Caldeira, K., and Matthews, H.D., Science, 2010, vol. 329, no. 5997, p. 1330.
Pontzen, F., Liebner, W., Gronemann, V., Rothaemel, M., and Ahlers, B., Catal. Today, 2011, vol. 171, no. 1, p. 242.
Ansari, M.B., and Park, S.-E., Energy Environ. Sci., 2012, vol. 5, no. 11, p. 9419.
Raudaskoski, R., Turpeinen, E., Lenkkeri, R., Pongrácz, E., and Keiski, R.L., Catal. Today, 2009, vol. 144, no. 3, p. 318.
Räuchle, K., Plass, L., Wernicke, H.-J., and Bertau, M., Energy Technol., 2016, vol. 4, no. 1, p. 193.
Inui, T., Hara, H., Takeguchi, T., and Kim, J.-B., Catal. Today, 1997, vol. 36, no. 1, p. 25.
Koizumi, N., Jiang, X., Kugai, J., and Song, C., Catal. Today, 2012, vol. 194, no. 1, p. 16.
Saito, M., Fujitani, T., Takeuchi, M., and Watanabe, T., Appl. Catal., A, 1996, vol. 138, no. 2, p. 311.
Arena, F., Italiano, G., Barbera, K., Bordiga, S., Bonura, G., Spadaro, L., and Frusteri, F., Appl.Catal., A, 2008, vol. 350, no. 1, p. 16.
Graciani, J., Mudiyanselage, K., Xu, F., Baber, A.E., Evans, J., Senanayake, S.D., Stacchiola, D.J., Liu, P., Hrbek, J., Sanz, J.F., and Rodriguez, J.A., Science, 2014, vol. 345, no. 6196, p. 546.
Sahibzada, M., Chem. Eng. Res. Des., 2000, vol. 78, no. 7, p. 943.
Bahruji, H., Bowker, M., Hutchings, G., Dimitratos, N., Wells, P., Gibson, E., Jones, W., Brookes, C., Morgan, D., and Lalev, G., J. Catal., 2016, vol. 343, no. 1, p. 133.
Chiavassa, D.L., Barrandeguy, J., Bonivardi, A.L., and Baltanás, M.A., Catal. Today, 2008, vols. 133–135, no. 1, p. 780.
Liang, X.-L., Dong, X., Lin, G.-D., and Zhang, H.-B., Appl. Catal., B, 2009, vol. 88, no. 3, p. 315.
Fujitani, T., Saito, M., Kanai, Y., Watanabe, T., Nakamura, J., and Uchijima, T., Appl.Catal., A, 1995, vol. 125, no. 2, p. 199.
Shao, C., Fan, L., Fujimoto, K., and Iwasawa, Y., Appl. Catal., A, 1995, vol. 128, no. 1, p. 1.
Collins, S.E., Chiavassa, D.L., Bonivardi, A.L., and Baltanás, M.A., Catal. Lett., 2005, vol. 103, no. 1, p. 83.
Zhang, L., Pan, L., Ni, C., Sun, T., Zhao, S., Wang, S., Wang, A., and Hu, Y., Int. J. Hydrogen Energy, 2013, vol. 38, no. 11, p. 4397.
Sonneveld, E.J. and Visser, J.W., J. Appl. Crystallogr., 1975, vol. 8, no. 1, p. 1.
Ahouari, H., Soualah, A., Le Valant, A., Pinard, L., Magnoux, P., and Pouilloux, Y., React. Kinet. Mech. Cat., 2013, vol. 110, no. 1, p. 131.
Zhang, B., Hui, S., Zhang, S., Ji, Y., Li, W., and Fang, D., J. Nat. Gas Chem., 2012, vol. 21, no. 5, p. 563.
Huang, C., Chen, S., Fei, X., Liu, D., and Zhang, Y., Catalysts, 2015, vol. 5, no. 4, p. 1846.
Schuyten, S., Guerrero, S., Miller, J.T., Shibata, T., and Wolf, E.E., Appl. Catal., A, 2009, vol. 352, no. 1, p. 133.
Mierczynski, P., Ciesielski, R., Kedziora, A., Shtyka, O., and Maniecki, T.P., Fibre Chem., 2016, vol. 48, no. 4, p. 271.
Jiang, X., Koizumi, N., Guo, X., and Song, C., Appl. Catal., B, 2015, vols. 170–171, no. 0, p. 173.
Jiang, X., Wang, X., Nie, X., Koizumi, N., Guo, X., and Song, C., Catal. Today, 2018, vol. 316, no. 1, p. 62.
Hu, B., Yin, Y., Liu, G., Chen, S., Hong, X., and Tsang, S.C.E., J. Catal., 2018, vol. 359, no. 1, p. 17.
Melián-Cabrera, I., López Granados, M., and Fierro, J.L.G., Catal. Lett., 2002, vol. 79, no. 1, p. 165.
Author information
Authors and Affiliations
Corresponding author
Additional information
Abbreviations: RWGS, reverse water gas shift reaction; TPR-H2, H2 temperature-programmed reduction; TCD, a thermal conductivity detector; TPD-NH3, NH3 temperature programmed desorption; SEM, scanning electron microscopy; EDS – energy dispersive X-ray spectroscopy; GC, gas chromatography; XRD, X-ray diffraction; FT-IR, Fourier-transform infrared spectroscopy.
Rights and permissions
About this article
Cite this article
Ciesielski, R., Shtyka, O., Zakrzewski, M. et al. Mechanistic Studies of Methanol Synthesis Reaction over Cu and Pd–Cu Catalysts. Kinet Catal 61, 623–630 (2020). https://doi.org/10.1134/S0023158420040035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158420040035